Publications
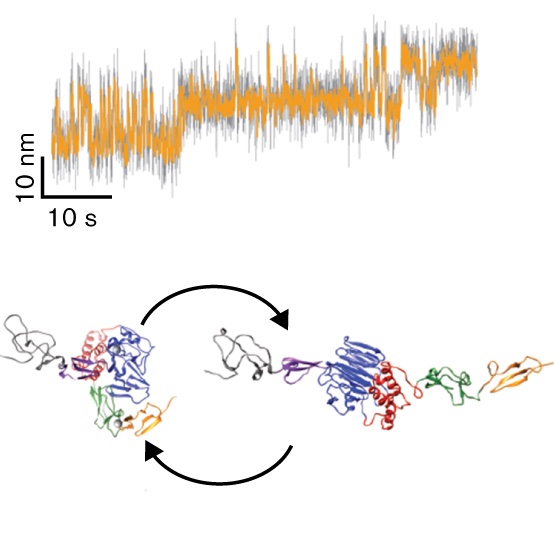 A conformational transition of the D'D3 domain primes von Willebrand factor for multimerization
A conformational transition of the D'D3 domain primes von Willebrand factor for multimerization
Sophia Gruber, Achim Löf, Adina Hausch, Fabian Kutzki, Res Jöhr, Tobias Obser, Gesa König, Reinhard Schneppenheim, Camilo Aponte-Santamaría, Frauke Gräter, Maria A. Brehm, Martin Benoit and Jan Lipfert
Blood Advances, September 2022, doi: 10.1182/bloodadvances.2022006978
Von Willebrand factor (VWF) is a multimeric plasma glycoprotein that is critically involved in hemostasis. Biosynthesis of long VWF concatemers in the endoplasmic reticulum and the trans-Golgi is still not fully understood. We use the single-molecule force spectroscopy technique magnetic tweezers to analyze a previously hypothesized conformational change in the D'D3 domain crucial for VWF multimerization. We find that... more PDF
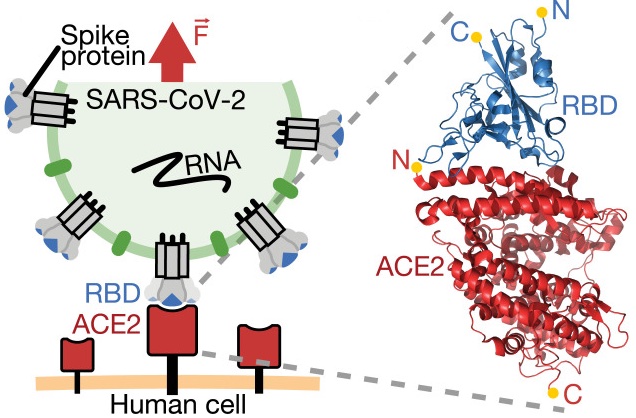 A tethered ligand assay to probe SARS-CoV-2:ACE2 interactions
A tethered ligand assay to probe SARS-CoV-2:ACE2 interactions
Magnus S. Bauer, Sophia Gruber, Adina Hausch, Priscila S.F.C. Gomes, Lukas F. Milles, Thomas Nicolaus, Leonard C .Schendel, Pilar López Navajas, Erik Procko, Daniel Lietha, Marcelo C.R. Melo, Rafael C. Bernardi, Hermann E. Gaub and Jan Lipfert
PNAS, April 2022, doi: 10.1073/pnas.2114397119
SignificanceIn the dynamic environment of the airways, where SARS-CoV-2 infections are initiated by binding to human host receptor ACE2, mechanical stability of the viral attachment is a crucial fitness advantage. Using single-molecule force spectroscopy techniques... more PDF
 Atomic Force Microscopy-Based Force Spectroscopy and Multiparametric Imaging of Biomolecular and Cellular Systems
Atomic Force Microscopy-Based Force Spectroscopy and Multiparametric Imaging of Biomolecular and Cellular Systems
Daniel J. Müller, Andra C. Dumitru, Cristina Lo Giudice, Hermann E. Gaub, Peter Hinterdorfer, Gerhard Hummer, James J. De Yoreo, Yves F. Dufrêne and David Alsteens
Chem Rev. October 2021, DOI: 10.1021/acs.chemrev.0c00617
During the last three decades, a series of key technological improvements turned atomic force microscopy (AFM) into a nanoscopic laboratory to directly observe and chemically characterize molecular and cell biological systems under physiological conditions. Here, we review key technological improvements that have established AFM as an analytical tool to observe and quantify native biological systems... more PDF
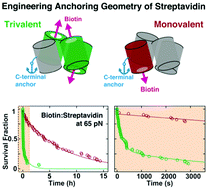
Designed anchoring geometries determine lifetimes of biotin–streptavidin bonds under constant load and enable ultra-stable coupling
Sophia Gruber, Achim Löf, Steffen M. Sedlak, Martin Benoit, Hermann E. Gaub and Jan Lipfert
Nanoscale, October 2020, DOI: 10.1039/d0nr03665j
The small molecule biotin and the homotetrameric protein streptavidin (SA) form a stable and robust complex that plays a pivotal role in many biotechnological and medical applications. In particular, the SA–biotin linkage is frequently used in single-molecule force spectroscopy (SMFS) experiments. Recent data suggest that SA–biotin bonds show strong directional dependence and a broad range of multi-exponential lifetimes under load. Here, we investigate engineered SA variants... more PDF
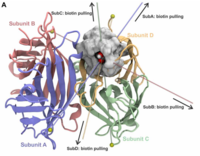 Streptavidin/biotin: Tethering geometry defines unbinding mechanics
Streptavidin/biotin: Tethering geometry defines unbinding mechanics
Steffen M. Sedlak, Leonard C. Schendel, Hermann E. Gaub and Rafael C. Bernardi
Science Advances, March 2020, DOI: 10.1126/sciadv.aay5999
Macromolecules tend to respond to applied forces in many different ways. Chemistry at high shear forces can be intriguing, with relatively soft bonds becoming very stiff in specific force-loading geometries. Largely used in bionanotechnology, an important case is the streptavidin (SA)/biotin interaction. Although SA’s four subunits have the same affinity, we find that the forces required to break the SA/biotin bond depend strongly on the attachment geometry... more PDF Presse
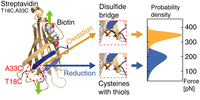 Switchable reinforced streptavidin
Switchable reinforced streptavidin
Leonard C. Schendel, Steffen M. Sedlak and Hermann E. Gaub
Nanoscale, March 2020, DOI: 10.1039/d0nr00265h
The complex of the small molecule biotin and the homotetrameric protein streptavidin is key to a broad range of biotechnological applications. Therefore, the behavior of this extraordinarily high-affinity interaction under mechanical force is intensively studied by single-molecule force spectroscopy. Recently,
steered molecular dynamics simulations have identified a low force pathway for the dissociation of biotin from streptavidin, which involves partial unfolding of the N-terminal β-sheet structure of monovalent streptavidin’s functional subunit... more PDF
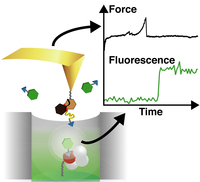 Single‐Molecule Manipulation in Zero‐Mode Waveguides
Single‐Molecule Manipulation in Zero‐Mode Waveguides
Leonard C. Schendel, Magnus S. Bauer, Steffen M. Sedlak, Hermann E. Gaub
Small Journal, March 2020, DOI: 10.1002/smll.201906740
The mechanobiology of receptor–ligand interactions and force‐induced enzymatic turnover can be revealed by simultaneous measurements of force response and fluorescence. Investigations at physiologically relevant high labeled substrate concentrations require total internal reflection fluorescence microscopy or zero mode waveguides (ZMWs), which are difficult to combine with atomic force microscopy (AFM). A fully automatized workflow is established to manipulate single molecules inside ZMWs autonomously with noninvasive cantilever tip localization. A protein model system comprising a receptor–ligand pair of streptavidin blocked with a biotin‐tagged ligand is introduced. ... more PDF
 Different Vinculin Binding Sites Use the Same Mechanism to Regulate Directional Force Transduction
Different Vinculin Binding Sites Use the Same Mechanism to Regulate Directional Force Transduction
Carleen Kluger, Lukas Braun, Steffen M.Sedlak, Diana A.Pippig, Magnus S.Bauer, Ken Miller, Lukas F.Milles, Hermann E. Gaub, Viola Vogel
Science Direct, Biophysical Journal , February 2020, https://doi.org/10.1016/j.bpj.2019.12.042
Vinculin is a universal adaptor protein that transiently reinforces the mechanical stability of adhesion complexes. It stabilizes mechanical connections that cells establish between the actomyosin cytoskeleton and the extracellular matrix via integrins or to neighboring cells via cadherins, yet little is known regarding its mechanical design. Vinculin binding sites (VBSs) from different nonhomologous actin-binding proteins use conserved helical motifs to associate with the vinculin head domain. We studied the mechanical stability of such complexes by pulling VBS peptides derived from talin, α-actinin, and Shigella IpaA out of the vinculin head domain... more PDF
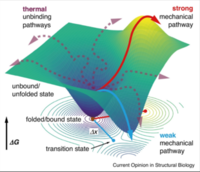 Extreme mechanical stability in protein complexes
Extreme mechanical stability in protein complexes
Lukas F Milles and Hermann E Gaub
Science Direct, February 2020, https://doi.org/10.1016/j.sbi.2019.11.012
Recently, non-covalent protein complexes and folds with extreme mechanical stabilities have been discovered. Various extracellular adhesin proteins of gram-positive bacteria exhibit complex rupture forces ranging from 800 pN in the case of cellulolytic bacteria to over 2000 pN withstood by pathogens adhering to their hosts. Here, we review and assess the mechanics of such systems, and discuss progress, as well as open questions regarding their biological function, and underlying molecular mechanisms — in particular the role of increased interaction lifetimes under mechanical load. These unexpected extreme strengths open an unchartered range of protein mechanics that can now be routinely probed by atomic force microscopy-based single-molecule force spectroscopy.... more PDF
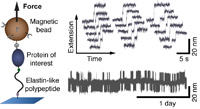 Multiplexed protein force spectroscopy reveals equilibrium protein folding dynamics and the low-force response of von Willebrand factor
Multiplexed protein force spectroscopy reveals equilibrium protein folding dynamics and the low-force response of von Willebrand factor
Achim Löf, Philipp U. Walker, Steffen M. Sedlak, Sophia Gruber, Tobias Obser, Maria A. Brehm, Martin Benoit, Jan Lipfert
PNAS, August 2019, https://doi.org/10.1073/pnas.1901794116
Single-molecule force spectroscopy has provided unprecedented insights into protein folding, force regulation, and function. So far, the field has relied primarily on atomic force microscope and optical tweezers assays that, while powerful, are limited in force resolution, throughput, and require feedback for constant force measurements. Here, we present a modular approach based on magnetic tweezers (MT) for highly multiplexed protein force spectroscopy. Our approach uses elastin-like polypeptide linkers for the specific attachment of proteins, requiring only short peptide tags on the protein of interest. The assay extends protein force spectroscopy into the low force (<1 pN) regime and enables parallel and ultra-stable measurements at constant forces... more PDF
 Mechanisms of Nanonewton Mechanostability in a Protein Complex Revealed by Molecular Dynamics Simulations and Single-Molecule Force Spectroscopy
Mechanisms of Nanonewton Mechanostability in a Protein Complex Revealed by Molecular Dynamics Simulations and Single-Molecule Force Spectroscopy
Rafael C. Bernardi, Ellis Durner, Constantin Schoeler, Klara H. Malinowska, Bruna G. Carvalho, Edward A. Bayer, Zaida Luthey-Schulten, Hermann E. Gaub, Michael A. Nash
JACS, August 2019, https://doi.org/10.1021/jacs.9b06776
Can molecular dynamics simulations predict the mechanical behavior of protein complexes? Can simulations decipher the role of protein domains of unknown func- tion in large macromolecular complexes? Here, we present a wide-sampling computational approach to demonstrate that molecular dynamics simulations, when carefully performed and combined with single-molecule atomic force spectroscopy experiments, can predict and explain the behavior of highly mechanostable protein complexes. As a test case, we studied a previously unreported homolog from Ruminococcus flavefaciens called X-module-Dockerin (XDoc) bound to its partner Cohesin (Coh)... more PDF
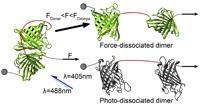 Dronpa: A Light-Switchable Fluorescent Protein for Opto-Biomechanics
Dronpa: A Light-Switchable Fluorescent Protein for Opto-Biomechanics
Res Jöhr, Magnus S. Bauer, Leonard C. Schendel, Carleen Kluger, and Hermann E. Gaub
Nano Lett, 2019, DOI: 10.1021/acs.nanolett.9b00639
Since the development of the green fluorescent protein, fluorescent proteins (FP) are indispensable tools in molecular biology. Some FPs change their structure under illumination, which affects their interaction with other biomolecules or proteins. In particular, FPs that are able to form switchable dimers became an important tool in the field of optogenetics. They are widely used for the investigation of signaling pathways, the control of surface recruitment, as well as enzyme and gene regulation. However, optogenetics did not yet develop tools for the investigation of biomechanical processes.... more PDF
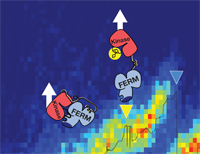 Structural and mechanistic insights into mechanoactivation of focal adhesion kinase
Structural and mechanistic insights into mechanoactivation of focal adhesion kinase
Magnus Sebastian Bauer, Fabian Baumann, Csaba Daday, Pilar Redondo, Ellis Durner, Markus Andreas Jobst, Lukas Frederik Milles, Davide Mercadante, Diana Angela Pippig, Hermann Eduard Gaub, Frauke Gräter, and Daniel Lietha
PNAS, first published March 15, 2019 https://doi.org/10.1073/pnas.1820567116
Focal adhesion kinase (FAK) is a key signaling molecule regulating cell adhesion, migration, and survival. FAK localizes into focal adhesion complexes formed at the cytoplasmic side of cell attachment to the ECM and is activated after force generation via actomyosin fibers attached to this complex. The mechanism of translating mechanical force into a biochemical signal is not understood, and it is not clear whether FAK is activated directly by force or downstream to the force signal. We use experimental and computational single-molecule force spectroscopy to probe the mechanical properties of FAK and examine whether force can trigger activation by inducing conformational changes in FAK.... more PDF
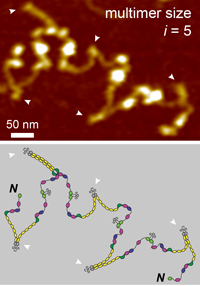 Advancing multimer analysis of von Willebrand factor by single-molecule AFM imaging
Advancing multimer analysis of von Willebrand factor by single-molecule AFM imaging
Achim Löf, Gesa König, Sonja Schneppenheim, Reinhard Schneppenheim, Martin Benoit, Ulrich Budde, Jochen P. Müller, and Maria A. Brehm
PLoS ONE 2019, 14(1):e0210963, DOI http://dx.plos.org/10.1371/journal.pone.0210963
The formation of hemostatic plugs at sites of vascular injury crucially involves the multimeric glycoprotein von Willebrand factor (VWF). VWF multimers are linear chains of N-terminally linked dimers. The latter are formed from monomers via formation of the C-terminal disulfide bonds Cys2771-Cys2773’, Cys2773-Cys2771’, and Cys2811-Cys2811’. Mutations in VWF that impair multimerization can lead to subtype 2A of the bleeding disorder von Willebrand Disease (VWD). Commonly, the multimer size distribution of VWF is assessed by electrophoretic multimer analysis. Here, we present atomic force microscopy (AFM) imaging as a method to determine the size distribution of VWF variants by direct visualization at the single-molecule level. We first validated our approach by investigating recombinant wildtype VWF and a previously studied mutant (p.Cys1099Tyr) that impairs N-terminal multimerization... more PDF
 Atomic force microscopy- based mechanobiology
Atomic force microscopy- based mechanobiology
Michael Krieg, Gotthold Fläschner, David Alsteens, Benjamin M. Gaub, Wouter H. Roos, Gijs J. L. Wuite, Hermann E. Gaub, Christoph Gerber, Yves F. Dufrêne and Daniel J. Müller
Nature Reviews Physicsvolume 1, pages41–57 (2019), DOI https://doi.org/10.1038/s42254-018-0001-7
Mechanobiology emerges at the crossroads of medicine, biology, biophysics and engineering and describes how the responses of proteins, cells, tissues and organs to mechanical cues contribute to development, differentiation, physiology and disease. The grand challenge in mechanobiology is to quantify how biological systems sense, transduce, respond and apply mechanical signals. Over the past three decades, atomic force microscopy (AFM) has emerged as a key platform enabling the simultaneous morphological and mechanical characterization of living biological systems... more PDF
 DNA-free directed assembly in single-molecule cut-and-paste
DNA-free directed assembly in single-molecule cut-and-paste
Katherine R. Erlich, Steffen M. Sedlak, Markus A. Jobst, Lukas F. Milles and Hermann E. Gaub
Nanoscale, December 2018, doi:10.1039/C8NR08636B
Single-molecule cut-and-paste facilitates bottom-up directed assembly of nanoscale biomolecular networks in defined geometries and enables analysis with spatio-temporal resolution. However, arrangement of diverse molecules of interest requires versatile handling systems. The novel DNA-free, genetically encodable scheme described here utilises an orthogonal handling strategy to promote arrangement of enzymes and enzyme networks... more PDF
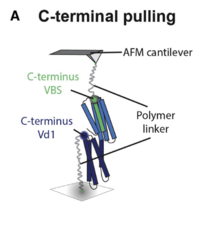
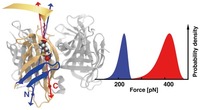 Direction Matters: Monovalent Streptavidin/Biotin Complex under Load
Direction Matters: Monovalent Streptavidin/Biotin Complex under Load
Steffen M. Sedlak, Leonard C. Schendel, Marcelo C. R. Melo, Diana A. Pippig, Zaida Luthey-Schulten, Hermann E. Gaub, and Rafael C. Bernardi
Nano Lett., October 2018, doi:10.1021/acs.nanolett.8b04045
Novel site-specific attachment strategies combined with improvements of computational resources enable new insights into the mechanics of the monovalent biotin/streptavidin complex under load and forced us to rethink the diversity of rupture forces reported in the literature. We discovered that the mechanical stability of this complex depends strongly on the geometry in which force is applied. By atomic force microscopy-based single molecule force spectroscopy we found unbinding of biotin to occur beyond 400 pN at force loading rates of 10 nN/s when monovalent streptavidin was tethered at its C-terminus... more PDF
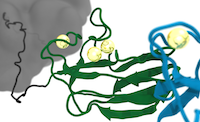 Calcium stabilizes the strongest protein fold
Calcium stabilizes the strongest protein fold
Lukas F. Milles, Eduard M. Unterauer, Thomas Nicolaus, Hermann E. Gaub
Nature Communications, November 2018, DOI: 10.1038/s41467-018-07145-6
Staphylococcal pathogens adhere to their human targets with exceptional resilience to mechanical stress, some propagating force to the bacterium via small, Ig-like folds called B domains. We examine the mechanical stability of these folds using atomic force microscopy-based single-molecule force spectroscopy. The force required to unfold a single B domain is larger than 2 nN – the highest mechanostability of a protein to date by a large margin. B domains coordinate three calcium ions, which we identify as crucial for their extreme mechanical strength... more PDF
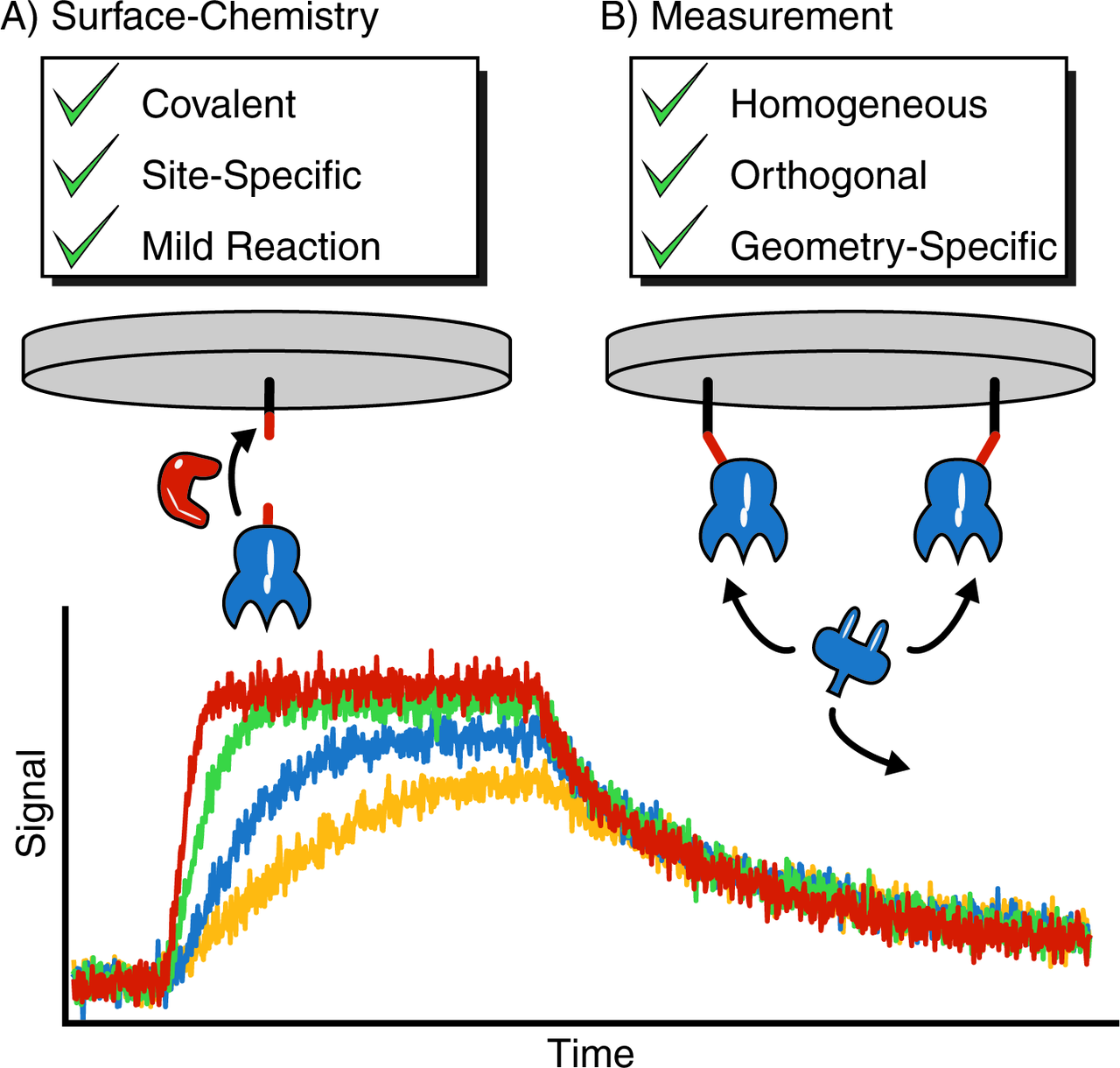 Enzyme-Mediated, Site-Specific Protein Coupling Strategies for Surface-Based Binding Assays
Enzyme-Mediated, Site-Specific Protein Coupling Strategies for Surface-Based Binding Assays
Wolfgang Ott, Ellis Durner, and Hermann E. Gaub
Angewandte Chemie International Edition, September 2018, DOI: 10.1002/anie.201805034
Covalent surface immobilization of proteins for binding assays is typically performed non-specifically via lysine residues. However, receptors that either have lysines near their
binding pockets, or whose presence at the sensor surface is electrostatically disfavoured, can be hard to probe. To overcome these limitations and to improve the homogeneity of surface functionalization, we adapted and optimized three different enzymatic coupling strategies (4’-phosphopantetheinyl transferase, sortase A, and asparaginyl endopeptidase) for biolayer interferometry surface modification... more PDF
 Covalent Immobilization of Proteins for the Single Molecule Force Spectroscopy
Covalent Immobilization of Proteins for the Single Molecule Force Spectroscopy
Tanja D. Becke, Stefan Ness, Stefanie Sudhop, Hermann E. Gaub, Markus Hilleringmann, Arndt F. Schilling, Hauke Clausen-Schaumann
Journal of Visualized Experiments, September 2018, DOI: 10.3791/58167
In recent years, atomic force microscopy (AFM) based single molecule force spectroscopy (SMFS) extended our understanding of molecular properties and functions. It gave us the opportunity to explore a multiplicity of biophysical mechanisms, e.g., how bacterial adhesins bind to host surface receptors in more detail. Among other factors, the success of SMFS experiments depends on the functional and native immobilization of the biomolecules of interest on solid surfaces and AFM tips... more PDF
 Ligand Binding Stabilizes Cellulosomal Cohesins as Revealed by AFM-based Single-Molecule Force Spectroscopy
Ligand Binding Stabilizes Cellulosomal Cohesins as Revealed by AFM-based Single-Molecule Force Spectroscopy
Tobias Verdorfer and Hermann E. Gaub
Scientific Reports, 2018, DOI: 10.1038/s41598-018-27085-x
The cohesin-dockerin receptor-ligand family is the key element in the formation of multi-enzyme lignocellulose-digesting extracellular complexes called cellulosomes. Changes in a receptor protein upon binding of a ligand - commonly referred to as allostery - are not just essential for signalling, but may also alter the overall mechanical stability of a protein receptor. Here, we measured the change in mechanical stability of a library of cohesin receptor domains upon binding of their dockerin ligands in a multiplexed atomic force microscopy-based single-molecule force spectroscopy experiment... more PDF
 Force-Induced Unravelling of DNA Origami
Force-Induced Unravelling of DNA Origami
Megan C. Engel, David M. Smith, Markus A. Jobst, Martin Sajfutdinow, Tim Liedl, Flavio Romano, Lorenzo Rovigatti, Ard A. Louis, and Jonathan P. K. Doye
ACS Nano, May 2018, DOI: 10.1021/acsnano.8b01844
The mechanical properties of DNA nanostructures are of widespread interest as applications that exploit their stability under constant or intermittent external forces become increasingly common. We explore the force response of DNA origami in comprehensive detail by combining AFM single molecule force spectroscopy experiments with simulations using oxDNA, a coarse-grained model of DNA at the nucleotide level, to study the unravelling of an iconic origami system: the Rothemund tile. We contrast the force-induced melting of the tile with simulations of an origami 10-helix bundle. Finally, we simulate a recently proposed origami biosensor, whose function takes advantage of...more PDF
 Molecular mechanism of extreme mechanostability in a pathogen adhesin
Molecular mechanism of extreme mechanostability in a pathogen adhesin
Lukas F. Milles, Klaus Schulten, Hermann E. Gaub, Rafael C. Bernardi
Science March 2018, DOI: 10.1126/science.aar2094
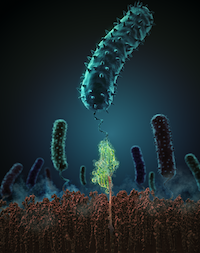
High resilience to mechanical stress is key when pathogens adhere to their target and initiate infection. Using atomic force microscopy–based single-molecule force spectroscopy, we explored the mechanical stability of the prototypical staphylococcal adhesin SdrG, which targets a short peptide from human fibrinogen β. Steered molecular dynamics simulations revealed, and single-molecule force spectroscopy experiments confirmed, the mechanism by which this complex withstands forces of over 2 nanonewtons, a regime previously associated with the strength of a covalent bond. The target peptide, confined in a screwlike manner in the binding pocket of SdrG, distributes forces mainly toward the peptide backbone through an intricate hydrogen bond network. Thus, these adhesins can attach to their target with exceptionally resilient mechanostability, virtually independent of peptide side chains...more PDF Perspectives PDF und SI
 Monodisperse measurement of the biotinstreptavidin interaction strength in a welldefined pulling geometry
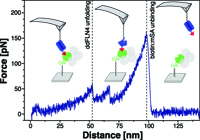
Monodisperse measurement of the biotinstreptavidin interaction strength in a welldefined pulling geometry
Steffen M. Sedlak, Magnus S. Bauer, Carleen Kluger, Leonard C. Schendel, Lukas F. Milles, Diana A. Pippig, Hermann E. Gaub*
PLOS ONE December 2017, https://doi.org/10.1371/journal.pone.0188722
The widely used interaction of the homotetramer streptavidin with the small molecule biotin has been intensively studied by force spectroscopy and has become a model system for receptor ligand interaction. However, streptavidin’s tetravalency results in diverse force propagation pathways through the different binding interfaces. This multiplicity gives rise to polydisperse force spectroscopy data. Here, we present an engineered monovalent streptavidin tetramer with a single cysteine in its functional subunit that allows for site-specific immobilization of the molecule, orthogonal to biotin binding...more PDF
 Combining in Vitro and in Silico Single-Molecule Force Spectroscopy to Characterize and Tune Cellulosomal Scaffoldin Mechanics
Combining in Vitro and in Silico Single-Molecule Force Spectroscopy to Characterize and Tune Cellulosomal Scaffoldin Mechanics
Tobias Verdorfer, Rafael C. Bernardi, Aylin Meinhold, Wolfgang Ott, Zaida Luthey-Schulten, Michael A. Nash, and Hermann E. Gaub
JACS October 2017, DOI: 10.1021/jacs.7b07574
Cellulosomes are polyprotein machineries that efficiently degrade cellulosic material. Crucial to their function are scaffolds consisting of highly homologous cohesin domains, which serve a dual role by coordinating a multiplicity of enzymes as well as anchoring the microbe to its substrate. Here we combined two approaches to elucidate the mechanical properties of the main scaffold ScaA of Acetivibrio cellulolyticus. A newly developed parallelized one-pot in vitro transcription−translation and protein pull-down protocol enabled high-throughputatomic force microscopy (AFM)-based single-molecule force spectroscopy (SMFS) measurements of all cohesins from ScaA with a single cantilever...more PDF

Strep-Tag II and Monovalent Strep-Tactin as Novel Handles in Single-Molecule Cut-and-Paste
Katherine R. Erlich, Fabian Baumann, Diana A. Pippig, and Hermann E. Gaub
Small Methods July 2017, DOI: 10.1002/smtd.201700169
Directed spatial assembly of single molecules on a surface presents an opportunity to precisely control the positioning, density, and geometry of molecules of interest within an ensemble. In contrast to bulk averaging, this enables detection and analysis of individual behavior within such a designed ensemble. The atomic force microscopy (AFM)-based technique of single-molecule cut-and-paste (SMC&P) facilitates the arrangement of a variety of biomolecules on a surface through different handling strategies...more PDF
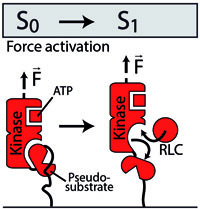
Increasing evidence of mechanical force as a functional regulator in smooth muscle myosin light chain kinase
Fabian Baumann, Magnus Sebastian Bauer, Martin Rees, Alexander Alexandrovich, Mathias Gautel, Diana Angela Pippig, Hermann Eduard Gaub,
eLIFE, 11 July 2017, DOI: 10.7554/eLife.26473.001
Mechanosensitive proteins are key players in cytoskeletal remodeling, muscle contraction, cell migration and differentiation processes. Smooth muscle myosin light chain kinase (smMLCK) is a member of a diverse group of serine/threonine kinases that feature cytoskeletal association. Its catalytic activity is triggered by a conformational change upon Ca2+/calmodulin (Ca2+/CaM) binding....more PDF
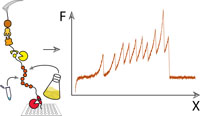
Post-Translational Sortase-Mediated Attachment of High-Strength
Force Spectroscopy Handles
Ellis Durner, Wolfgang Ott, Michael A. Nash and Hermann E. Gaub, ACS Omega, 30 June 2017, DOI: 10.1021/acsomega.7b00478
Single-molecule force spectroscopy greatly benefits from site-specific surface immobilization and specific probing with a functionalized cantilever.Here, we describe a streamlined approach to such experiments by covalently attaching mechanically stable receptors onto proteins of interest (POI) to improve pickup efficiency and specificity. This platform provides improved throughput, allows precise control over the pulling geometry, and allows for multiple constructs to be probed with the same ligand-modified cantilever...more PDF
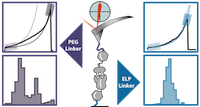
Elastin-like Polypeptide Linkers for Single-Molecule Force Spectroscopy
Wolfgang Ott, Markus A. Jobst, Magnus S. Bauer, Ellis Durner, Lukas F. Milles, Michael A. Nash, and Hermann E. Gaub, ACS Nano, 7 June 2017, DOI: 10.1021/acsnano.7b02694
Single-molecule force spectroscopy (SMFS) is by now well established as a standard technique in biophysics and mechanobiology. In recent years, the technique has benefitted greatly from new approaches to bioconjugation of proteins to surfaces. Indeed, optimized immobilization strategies for biomolecules and refined purification schemes are being steadily adapted and improved, which in turn has enhanced data quality. In many previously reported SMFS studies, poly(ethylene glycol) (PEG) was used to anchor molecules of interest to surfaces and/or cantilever tips. The limitation, however, is that PEG exhibits a well-known trans−trans−gauche to all-trans transition, ... more PDF
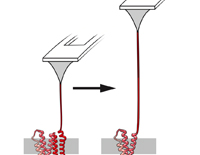 Membrane proteins scrambling through a folding landscape
Membrane proteins scrambling through a folding landscape
Daniel J. Müller and Hermann E. Gaub, Science Perspective, 3 March 2017 • VOL 355 ISSUE 6328, doi: 10.1126/science.aam8370.
Single-molecule force spectroscopy (SMFS) measures the extension of a molecule when subjected to force. The folding of a protein can be explored by pulling on one terminus to unfold it; upon relaxation, it may refold toward its native states. Transmembrane proteins typically unfold stepwise as structural segments (which can consist of parts of single or multiple secondary structures) are extracted from the membrane (see the figure, top panel). Once extracted, the unfolded segment can insert back into the membrane and fold toward the native protein structure. Complex proteins extracted from the membrane may refold back into the membrane but tend to misfold and require the assistance of chaperones, insertases, or both... more PDF
 Atomic force microscopy-based characterization and design of biointerfaces
Atomic force microscopy-based characterization and design of biointerfaces
David Alsteens, Hermann E. Gaub, Richard Newton, Moritz Pfreundschuh, Christoph Gerber and Daniel J. Müller, Nature Reviews,
doi: 10.1038/natrevmats.2017.8, Published online 14 Mar 2017,
Atomic force microscopy (AFM)-based methods have matured into a powerful nanoscopic platform, enabling the characterization of a wide range of biological and synthetic biointerfaces ranging from tissues, cells, membranes, proteins, nucleic acids and functional materials. Although the unprecedented signal-to-noise ratio of AFM enables the imaging of biological interfaces from the cellular to the molecular scale, AFM-based force spectroscopy allows their mechanical, chemical, conductive or electrostatic, and biological properties to be probed... more PDF
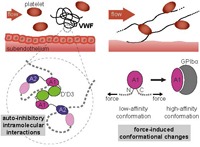 A Biophysical View on von Willebrand Factor Activation
A Biophysical View on von Willebrand Factor Activation
Achim Löf, Jochen P. Müller, and Maria A. Brehm, Journal of Cellular Physiology,
doi: 10.1002/jcp.25887, March 2017
The process of hemostatic plug formation at sites of vascular injury crucially relies on the large multimeric plasma glycoprotein von Willebrand factor (VWF) and its ability to recruit platelets to the damaged vessel wall via interaction of its A1 domain with platelet GPIbα. Under normal blood flow conditions, VWF multimers exhibit a very low binding affinity for platelets. Only when subjected to increased hydrodynamic forces, which primarily occur in connection with vascular injury, VWF can efficiently bind to platelets. This force-regulation of VWF’s hemostatic activity is not only highly intriguing from a biophysical perspective, but also of eminent physiological importance... more PDF
 Biophysical approaches promote advances in the understanding of von Willebrand factor processing and function
Biophysical approaches promote advances in the understanding of von Willebrand factor processing and function
Löf A., Müller J.P., Benoit M., Brehm M.A., Advances in Biological Regulation 2017, Vol 63, pages 81-91.
The large multimeric plasma glycoprotein von Willebrand factor (VWF) is essential for primary hemostasis by recruiting platelets to sites of vascular injury. VWF multimers respond to elevated hydrodynamic forces by elongation, thereby increasing their adhesiveness to platelets. Thus, the activation of VWF is force-induced, as is its inactivation. Due to these attributes, VWF is a highly interesting system from a biophysical point of view, and is well suited for investigation using biophysical approaches. Here, we give an overview on recent studies that predominantly employed biophysical methods to gain novel insights into multiple aspects of VWF: Electron microscopy was used to shed light on the domain structure of VWF and the mechanism of VWF secretion... more PDF
 Mechanical Stability of a High-Affinity Toxin Anchor from the Pathogen Clostridium perfringens
Mechanical Stability of a High-Affinity Toxin Anchor from the Pathogen Clostridium perfringens
Lukas F. Milles, Edward. A. Bayer, Michael A. Nash, Hermann E. Gaub, Journal of Physical Chemistry B - Klaus Schulten Memorial Issue, doi: 10.1021/acs.jpcb.6b09593, November 2016
The opportunistic pathogen Clostridium perfringens assembles its toxins and carbohydrate-active enzymes by the high-affinity cohesin-dockerin (Coh-Doc) interaction. Coh–Doc interactions characterized previously have shown considerable resilience toward mechanical stress. Here, we aimed to determine the mechanics of this interaction from C. perfringens in the context of a pathogen. Using atomic force microscopy based single-molecule force spectroscopy (AFM-SMFS) we probed the mechanical properties of the interaction of a dockerin from the μ-toxin with the GH84C X82 cohesin domain of C. perfringens. Most probable complex rupture forces were found to be approximately 60 pN... more PDF
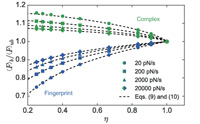 Biasing effects of receptor-ligand complexes on protein-unfolding statistics
Biasing effects of receptor-ligand complexes on protein-unfolding statistics
Constantin Schoeler, Tobias Verdorfer, Hermann E. Gaub, and Michael A. Nash, Physical Review, Vol. 94, Iss. 4, DOI:https://doi.org/10.1103/PhysRevE.94.042412, October 2016
Protein receptor-ligand pairs are increasingly used as specific molecular handles in single-molecule protein-unfolding experiments. Further, known marker domains, also referred to as fingerprints, provide unique unfolding signatures to identify specific single-molecule interactions, when receptor-ligand pairs themselves are investigated... more PDF
 Nanoscale Engineering of Designer Cellulosomes
Nanoscale Engineering of Designer Cellulosomes
Melissabye Gunnoo, Pierre-André Cazade, Albert Galera-Prat, Michael A. Nash,
Mirjam Czjzek, Marek Cieplak, Beatriz Alvarez , Marina Aguilar, Alon Karpol,
Hermann Gaub, Mariano Carrión-Vázquez, Edward A. Bayer, and Damien Thompson, Advanced Materials, 2016, 28, 5619–5647
doi: 10.1002/adma.201503948, Juli 2016
Biocatalysts showcase the upper limit obtainable for high-speed molecular processing and transformation. Efforts to engineer functionality in synthetic nanostructured materials are guided by the increasing knowledge of evolving architectures, which enable controlled molecular motion and precise molecular recognition. The cellulosome is a biological nanomachine, which, as a fundamental component of the plant-digestion machinery from bacterial cells, has a key potential role in the successful development of environmentally-friendly processes to produce biofuels and fine chemicals from the breakdown of biomass waste. Here, the progress toward so-called “designer cellulosomes”, which provide an elegant alternative to enzyme cocktails for lignocellulose breakdown, is reviewed.... more PDF
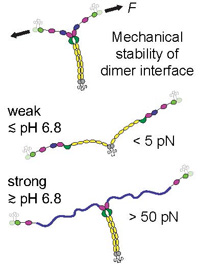 pH-Dependent Interactions in Dimers Govern the Mechanics and Structure of von Willebrand Factor
pH-Dependent Interactions in Dimers Govern the Mechanics and Structure of von Willebrand Factor
Jochen P. Müller, Achim Löf, Salomé Mielke, Tobias Obser, Linda K. Bruetzel, Willem Vanderlinden, Jan Lipfert, Reinhard Schneppenheim, and Martin Benoit, Biophysical Journal, Volume 111, Issue 2, Pages 312–322, doi:10.1016/j.bpj.2016.06.022, 26 July 2016,
Von Willebrand factor (VWF) is a multimeric plasma glycoprotein that is activated for hemostasis by increased hydrodynamic forces at sites of vascular injury. Here, we present data from atomic force microscopy-based single-molecule force measurements, atomic force microscopy imaging, and small-angle x-ray scattering to show that the structure and mechanics of VWF are governed by multiple pH-dependent interactions with opposite trends within dimeric subunits. In particular, the recently discovered strong intermonomer interaction, which induces a firmly closed conformation of dimers and crucially involves the D4 domain... more PDF
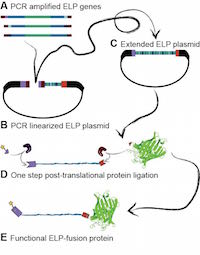
Sequence-Independent Cloning and Post-Translational Modification
of Repetitive Protein Polymers through Sortase and Sfp-Mediated
Enzymatic Ligation
Wolfgang Ott, Thomas Nicolaus, Hermann E. Gaub, and Michael A. Nash
Biomacromolecules, doi:10.1021/acs.biomac.5b01726, March 2016
Repetitive protein-based polymers are important for many applications in biotechnology and biomaterials development. Here we describe the sequential additive ligation of highly repetitive DNA sequences, their assembly into genes encoding protein–polymers with precisely tunable lengths and compositions, and their end-specific post-translational modification with organic dyes and fluorescent protein domains. Our new Golden Gate-based cloning approach relies on incorporation of only type IIS BsaI restriction enzyme recognition sites using PCR, which allowed us to install ybbR-peptide tags, Sortase c-tags, and cysteine residues... more PDF
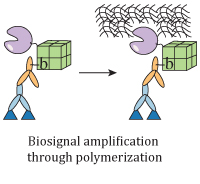
Enzyme- and affinity biomolecule-mediated polymerization systems for biological signal amplification and cell screening
Klara H. Malinowska, Michael A. Nash
Current Opinion in Biotechnology, doi:10.1016/j.copbio.2016.01.007, February 2016
Enzyme-mediated polymerization and polymerization-based signal amplification have emerged as two closely related techniques that are broadly applicable in the nanobio sciences. We review recent progress on polymerization systems mediated by biological molecules (e.g., affinity molecules and enzymes), and highlight newly developed formats and configurations of these systems to perform such tasks as non-instrumented biodetection, synthesis of core–shell nanomaterials, isolation of rare cells, and high-throughput screening... more PDF
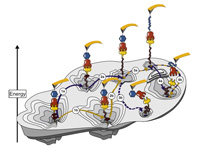
Single-molecule force spectroscopy on polyproteins and receptor–ligand complexes: The current toolbox
Wolfgang Ott, Markus A. Jobst, Constantin Schoeler, Hermann E. Gaub, Michael A. Nash
Journal of Structural Biology, doi:10.1016/j.jsb.2016.02.011, February 2016
Single-molecule force spectroscopy sheds light onto the free energy landscapes governing protein folding and molecular recognition. Since only a single molecule or single molecular complex is probed at any given point in time, the technique is capable of identifying low-probability conformations within a large ensemble of possibilities. It furthermore allows choosing certain unbinding pathways through careful selection of the... more PDF
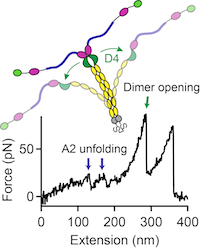
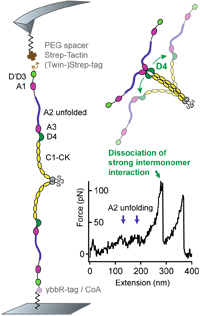
Force sensing by the vascular protein von Willebrand factor is tuned by a strong intermonomer interaction
Jochen P. Müller, Salomé Mielke, Achim Löf, Tobias Obser, Christof Beer, Linda K. Bruetzel, Diana A. Pippig, Willem Vanderlinden, Jan Lipfert, Reinhard Schneppenheim, Martin Benoit
PNAS, doi: 10.1073/pnas.1516214113, January 2016
The large plasma glycoprotein von Willebrand factor (VWF) senses hydrodynamic forces in the bloodstream and responds to elevated forces with abrupt elongation, thereby increasing its adhesiveness to platelets and collagen. Remarkably, forces on VWF are elevated at sites of vascular injury, where VWF’s hemostatic potential is important to mediate platelet aggregation and to recruit platelets to the subendothelial layer... more PDF
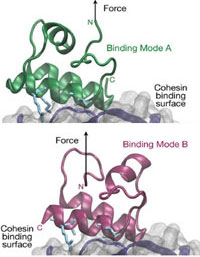 Resolving dual binding conformations of cellulosome cohesin-dockerin complexes using single-molecule force spectroscopy
Resolving dual binding conformations of cellulosome cohesin-dockerin complexes using single-molecule force spectroscopy
Markus A. Jobst, Lukas F. Milles, Constantin Schoeler, Wolfgang Ott, Daniel B. Fried, Edward A. Bayer, Hermann E. Gaub & Michael A. Nash
eLife, DOI:10.7554/eLife.10319.001, October 2015
Receptor-ligand pairs are ordinarily thought to interact through a lock and key mechanism, where a unique molecular conformation is formed upon binding. Contrary to this paradigm, cellulosomal cohesin-dockerin (Coh-Doc) pairs are believed to interact through redundant dual binding modes consisting of two distinct conformations. Here, we combined site- directed mutagenesis and single-molecule force spectroscopy (SMFS) to study the unbinding of Coh:Doc complexes under force. We designed Doc mutations to knock out each binding mode, and compared their single-molecule unfolding patterns as they were dissociated from... more PDF
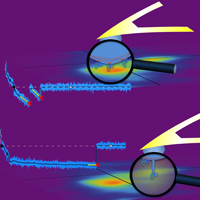 Decoding Cytoskeleton-Anchored and Non-Anchored Receptors from Single-Cell Adhesion Force Data
Decoding Cytoskeleton-Anchored and Non-Anchored Receptors from Single-Cell Adhesion Force Data
Ediz Sariisik, Cvetan Popov, Jochen P. Müller, Denitsa Docheva, Hauke Clausen-Schaumann and Martin Benoit
Biophys J. 2015 Oct 6;109(7):1330-3. doi: 10.1016/j.bpj.2015.07.048
Complementary to parameters established for cell-adhesion force curve analysis, we evaluated the slope before a force step together with the distance from the surface at which the step occurs and visualized the result in a two-dimensional density plot. This new tool allows detachment steps of long membrane tethers to be distinguished from shorter jumplike force steps.... more PDF
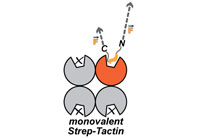 Monovalent Strep-Tactin for strong and site-specific tethering in nano spectroscopy
Monovalent Strep-Tactin for strong and site-specific tethering in nano spectroscopy
Fabian Baumann, Magnus S. Bauer, Lukas F. Milles, Alexander Alexandrovich, Hermann E. Gaub & Diana A. Pippig
Nature Nanotechnology, doi:10.1038/nnano.2015.231, October 2015
Strep-Tactin, an engineered form of streptavidin, binds avidly to the genetically encoded peptide Strep-tag II in a manner comparable to streptavidin binding to biotin. These interactions have been used in protein purification and detection applications. However, in single-molecule studies, for example using atomic force microscopy-based single-molecule force spectroscopy (AFM-SMFS), the tetravalency of these systems... more PDF
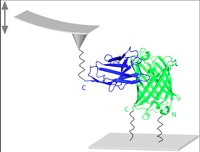 Energy profile of nanobody-GFP complex under force
Energy profile of nanobody-GFP complex under force
Kamila Klamecka, Philip M Severin, Lukas F. Milles, Hermann E. Gaub & Heinrich Leonhardt
Physical Biology, Volume 12, Number 5, doi:10.1088/1478-3975/12/5/056009, September 2015
Nanobodies (Nbs)-the smallest known fully functional and naturally occuring antigen-binding fragments-have attracted a lot of attention throughout the last two decades. Exploring their potential beyond the current use requires more detailed characterization of their binding forces as those cannot be directly derived from the binding affinities. Here we used atomic force microscope to measure rupture force of the Nb-green fluorescent protein (GFP) complex in various pulling geometries and derived the energy profile characterizing the interaction along the direction of the pulling force.... more PDF
 Mapping Mechanical Force Propagation through Biomolecular Complexes
Mapping Mechanical Force Propagation through Biomolecular Complexes
Constantin Schoeler, Rafael C. Bernardi, Klara H. Malinowska, Ellis Durner, Wolfgang Ott, Edward A. Bayer, Klaus Schulten, Michael A. Nash, and Hermann E. Gaub
Nano Lett., doi:10.1021/acs.nanolett.5b02727, August 2015
Here we employ single-molecule force spectroscopy with an atomic force microscope (AFM) and steered molecular dynamics (SMD) simulations to reveal force propagation pathways through a mechanically ultrastable multidomain cellulosome protein complex. We demonstrate a new combination of network-based... more PDF
 Quantifying Synergy, Thermostability, and Targeting of Cellulolytic Enzymes and Cellulosomes with Polymerization-Based Amplification
Quantifying Synergy, Thermostability, and Targeting of Cellulolytic Enzymes and Cellulosomes with Polymerization-Based Amplification
Klara H. Malinowska, Thomas Rind , Tobias Verdorfer, Hermann E. Gaub , and Michael A. Nash
Anal. Chem.; doi:10.1021/acs.analchem.5b00936, June 2015
We present a polymerization-based assay for determining the potency of cellulolytic enzyme formulations on pretreated biomass substrates. Our system relies on monitoring the autofluorescence of cellulose and measuring the... more PDF
 C-5 Propynyl Modifications Enhance the Mechanical Stability of DNA
C-5 Propynyl Modifications Enhance the Mechanical Stability of DNA
Daniela Aschenbrenner, Fabian Baumann, Lukas F. Milles, Dr. Diana A. Pippig, and Hermann E. Gaub
ChemPhysChem; doi:10.1002/cphc.201500193, May 2015
Increased thermal or mechanical stability of DNA duplexes is desired for many applications in nanotechnology or -medicine where DNA is used as a programmable building block. Modifications of pyrimidine bases are known to enhance thermal stability and have the advantage of standard base-pairing and... more PDF
 Tip localization of an atomic force microscope in transmission microscopy with nanoscale precision
Tip localization of an atomic force microscope in transmission microscopy with nanoscale precision
Fabian Baumann, Stephan F. Heucke, Diana A. Pippig and Hermann E. Gaub
Rev. Sci. Instrum.; http://dx.doi.org/10.1063/1.4915145, March 2015
Since the atomic force microscope (AFM) has evolved into a general purpose platform for mechanical experiments at the nanoscale, the need for a simple and generally applicable localization of the AFM cantilever in the reference frame of an optical microscope has grown. Molecular manipulations... more PDF
 A fast recoiling silk-like elastomer facilitates nanosecond nematocyst discharge
A fast recoiling silk-like elastomer facilitates nanosecond nematocyst discharge
Anna Beckmann, Senbo Xiao, Jochen P Müller, Davide Mercadante, Timm Nüchter, Niels Kröger, Florian Langhojer, Wolfgang Petrich, Thomas W Holstein, Martin Benoit, Frauke Gräter & Suat Özbek
BMC Biology. 2015, 13:3 , doi: 10.1186/s12915-014-0113-1, 16 January 2015
Here, we characterise Cnidoin, a novel elastic protein identified as a structural component of Hydra nematocysts. Cnidoin is expressed in nematocytes of all types and immunostainings revealed incorporation into capsule walls and tubules concomitant with minicollagens... more PDF
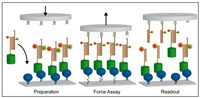 Parallel Force Assay for Protein-Protein Interactions
Parallel Force Assay for Protein-Protein Interactions
Daniela Aschenbrenner, Diana A. Pippig , Kamila Klamecka, Katja Limmer, Heinrich Leonhardt, Hermann E. Gaub
PLOSone, doi:10.1371/journal.pone.0115049, December 2014
Quantitative proteome research is greatly promoted by high-resolution parallel format assays. A characterization of protein complexes based on binding forces offers an unparalleled dynamic range and allows for the effective discrimination of non-specific interactions. Here we present a DNA-based Molecular Force Assay to quantify... more PDF
 Ultrastable cellulosome-adhesion complex tightens under load
Ultrastable cellulosome-adhesion complex tightens under load
Constantin Schoeler, Klara H. Malinowska, Rafael C. Bernardi, Lukas F. Milles, Markus A. Jobst, Ellis Durner, Wolfgang Ott, Daniel B. Fried, Edward A. Bayer, Klaus Schulten, Hermann E. Gaub & Michael A. Nash
Nature Communications, doi:10.1038/ncomms6635, December 2014
Challenging environments have guided nature in the development of ultrastable protein complexes. Specialized bacteria produce discrete multi-component protein networks called cellulosomes to effectively digest lignocellulosic biomass. While network assembly is enabled by protein interactions with ... more PDF
From genes to protein mechanics on a chip
Marcus Otten, Wolfgang Ott, Markus A Jobst, Lukas F Milles, Tobias Verdorfer, Diana A Pippig, Michael A Nash & Hermann E Gaub
Nature Methods, doi:10.1038/nmeth.3099, September 2014
Single-molecule force spectroscopy enables mechanical testing of individual proteins, but low experimental throughput limits the ability to screen constructs in parallel. We describe a microfluidic platform for on-chip expression, covalent surface attachment and measurement of single-molecule protein mechanical ... more PDF

Redox-Initiated Hydrogel System for Detection and Real- Time Imaging of Cellulolytic Enzyme Activity
Klara H. Malinowska, Tobias Verdorfer, Aylin Meinhold, Lukas F. Milles, Victor Funk, Hermann E. Gaub, and Michael A. Nash
ChemSusChem, doi:10.1002/cssc.201402428, August 2014
A hydrogel reagent signaling (HyReS) system converts oligosaccharides produced during biomass hydrolysis into a fluorescent hydrogel. This system for assaying cellulolytic enzyme activity serves as a versatile platform on both soluble and insoluble substrates. When combined with total internal reflection fluorescence microscopy, it provides a spatially resolved method for chemical imaging of .... more PDF
Functionalization of Cantilever Tips with Nucleotides by
the Phosphoramidite Method
Ralf David, Matthias Erdmann, Ann R. Fornof, and Hermann E. Gaub
ChemMedChem 2014, 9, 2049 – 2051, DOI: 10.1002/cmdc.201402165, July 2014
In atomic force microscopy (AFM) a sharp cantilever tip is used to scan surfaces at the atomic level. One further application is force spectroscopy, in which force–distance curves between binding partners located on the cantilever and substrate surface are determined. This requires specifically immobilized molecules. Herein we describe the covalent binding of single adenosine and thymidine nucleotides on an amino-PEGylated cantilever tip by the phosphoramidite method. Force–distance curves between these cantilever tips and gold surfaces were recorded....more PDF
Protein–DNA Chimeras for Nano Assembly
Diana A. Pippig, Fabian Baumann, Mathias Strackharn, Daniela Aschenbrenner and Hermann E. Gaub
ACS Nano, Article ASAP, DOI:10.1021/nn501644w, June 2014
In synthetic biology, “understanding by building” requires exquisite control of the molecular constituents and their spatial organization. Site-specific coupling of DNA to proteins allows arrangement of different protein functionalities with emergent properties by self-assembly on origami-like DNA scaffolds .... more PDF

Detection of Thermoresponsive Polymer Phase Transition in Dilute Low-Volume Format by Microscale Thermophoretic Depletion
M. Wolff, D. Braun and M.A. Nash
Anal. Chem., DOI:10.1021/ac5008283, May 2014
Environmentally responsive polymers are becoming increasingly important in the biomaterials field for use as diagnostic reagents, drug carriers, and tissue engineering scaffolds. Characterizing polymer phase transitions by cloud point curves typically requires large milliliter volumes of sample at high micromolar solution concentrations. Here we present a method based on quantification of thermophoretic Soret diffusion that allows determination of polymer phase transitions using only 1 μL of liquid at dilute nanomolar concentrations, effectively reducing the amount of sample required by a factor of 106. We prepared an .... more PDF
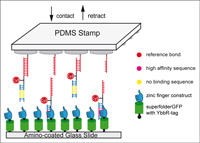
A Force-Based, Parallel Assay for the Quantification of Protein-DNA Interactions
Katja Limmer, Diana A. Pippig, Daniela Aschenbrenner, Hermann E. Gaub
PLOS ONE, DOI: 10.1371/journal.pone.0089626, February 2014
Analysis of transcription factor binding to DNA sequences is of utmost importance to understand the intricate regulatory mechanisms that underlie gene expression. Several techniques exist that quantify DNA-protein affinity .... more PDF

Placing individual molecules in the center of nanoapertures
Heucke S.F., Baumann F., Acuna G.P., Severin P., Stahl S.W., Strackharn M., Stein I., Altpeter P., Tinnefeld P. and Gaub H.E.
Nano Letters, Volume 14, Issue 2, DOI: 10.1021/nl401517a, February 2014
While nanophotonic devices are unfolding their potential for single-molecule fluorescence studies, metallic quenching and steric hindrance, occurring within these structures, raise the desire for site-specific immobilization of the molecule of interest. Here, we refine the single-molecule cut-and-paste technique by optical superresolution routines to immobilize single fluorescent molecules in the center of nanoapertures. By comparing their fluorescence lifetime and intensity to stochastically immobilized fluorophores, we characterize the .... more PDF
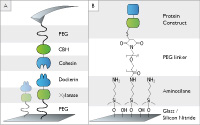 Investigating Receptor-ligand Systems of the Cellulosome with AFM-based Single-molecule Force Spectroscopy
Investigating Receptor-ligand Systems of the Cellulosome with AFM-based Single-molecule Force Spectroscopy
Markus A. Jobst, Constantin Schoeler, Klara Malinowska, Michael A. Nash
JoVE, DOI: 10.3791/50950, 20 December 2013
Cellulosomes are multienzyme complexes designed for digesting cellulose. AFM-based SMFS was used to study the mechanical properties and folding .... more PDF
movie
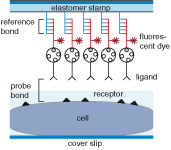 Stamping Vital Cells—a Force-Based Ligand Receptor Assay
Stamping Vital Cells—a Force-Based Ligand Receptor Assay
Uta Wienken and Hermann E. Gaub
Biophysical Journal, Volume 105, Issue 12, 2687-2694, 17 December 2013
Gaining information about receptor profiles on cells, and subsequently finding the most efficient ligands for these signaling receptors, remain challenging tasks in stem cell and cancer research as well as drug development. We introduce a live-cell method .... more PDF
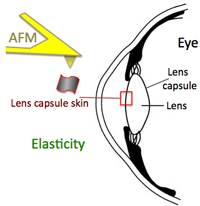 Increase in lens capsule stiffness caused by vital dyes
Increase in lens capsule stiffness caused by vital dyes
Christos Haritoglou, MD, Stephan Mauell, MSc, Ricarda G. Schumann, MD, Paul B. Henrich, MD, Armin Wolf, MD, Marcus Kernt, MD, Martin Benoit, PhD
J Cataract Refract Surg 2013; 39:1749–1752 Q 2013 ASCRS and ESCRS, doi:10.1016/j.jcrs.2013.02.057
Cataract surgery was among the first ophthalmic surgical procedures in which dyes were introduced to assist the surgeon in more challenging cases, such as eyes with mature cataract. In mature white cataracts, a controlled capsulorhexis of the anterior capsule is often difficult to performdue to absence of the red fundus reflex. .... more PDF
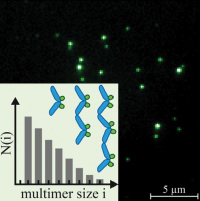 Exponential size distribution of von Willebrand factor.
Exponential size distribution of von Willebrand factor.
Lippok S, Obser T, Müller JP, Stierle VK, Benoit M, Budde U, Schneppenheim R, Rädler JO.
Biophys J. 2013 Sep 3;105(5):1208-16. doi: 10.1016/j.bpj.2013.07.037.
Von Willebrand Factor (VWF) is a multimeric protein crucial for hemostasis. Under shear flow, it acts as a mechanosensor responding with a size-dependent globule-stretch transition to increasing shear rates. Here, we quantify for the first time, to our knowledge, the size distribution of recombinant VWF and VWF-eGFP using a multilateral approach that involves quantitative gel analysis, fluorescence correlation spectroscopy, and total internal reflection fluorescence microscopy... more PDF
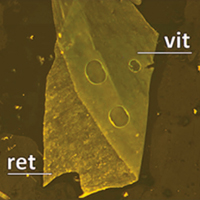 Vital dyes increase the rigidity of the internal limiting membrane
Vital dyes increase the rigidity of the internal limiting membrane
Haritoglou C, Mauell S, Benoit M, Schumann RG, Henrich PB, Wolf A and Kampik A
Eye advance online publication, 16 August 2013; doi:10.1038/eye.2013.178
To assess the stiffness of the natural human internal limiting membrane (ILM) and evaluate potential changes of the mechanical properties following staining with brilliant blue (BB) and indocyanine green (ICG).
Methods Unstained ILM specimens were obtained during ophthalmic surgical procedures. After removal, the specimens were dissected into five parts. .... more PDF
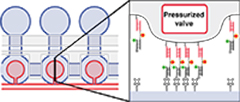 Protein-DNA force assay in a microfluidic format
Protein-DNA force assay in a microfluidic format
Otten M., Wolf P. and Gaub H.E.
Lab Chip, DOI: 10.1039/C3LC50830G, Aug. 2013
The detailed study of protein–DNA interactions is a core effort to elucidate physiological processes, including gene regulation, DNA repair and the immune response. The molecular force .... more PDF
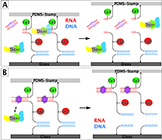 Sequence-specific inhibition of Dicer measured with a force-based microarray for RNA ligands
Sequence-specific inhibition of Dicer measured with a force-based microarray for RNA ligands
Limmer K., Aschenbrenner D. and Hermann Gaub H.E.
Nucleic Acids Research, Vol. 41, No. 6 e69, doi:10.1093/nar/gks1455 (2013)
Malfunction of protein translation causes many severe diseases, and suitable correction strategies may become the basis of effective therapies. One major regulatory element of protein translation is the nuclease Dicer that cuts double-stranded RNA.....more PDF
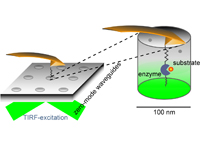 Nanoapertures for AFM-Based Single-Molecule Force Spectroscopy
Nanoapertures for AFM-Based Single-Molecule Force Spectroscopy
Heucke S.F., Puchner E.M., Stahl S.W., Holleitner A.W., Gaub H.E. and Tinnefeld P.
Int. J. Nanotechnology 10, No. 5/6/7, 607–619 (2013)
Simultaneous single-molecule force spectroscopy and microfluorescence binding measurements are often hampered by background fluorescence from the bulk. Zero-Mode Waveguides (ZMW) restrict the excited volume but require a spectial design, which allows the tip of the force probing cantilever to protrude into....... more PDF
 Effects of Cytosine Hydroxymethylation on DNA Strand Separation
Effects of Cytosine Hydroxymethylation on DNA Strand Separation
Philip M. D. Severin, Xueqing Zou, Klaus Schulten and Hermann E. Gaub
Biophysical Journal, Volume 104 January 2013 208–215, DOI: 10.1016/j.bpj.2012.11.013
Cytosine hydroxymethylation is an epigenetic control factor in higher organisms. New discoveries of the biological roles of hydroxymethylation serve to raise questions about how this epigenetic modification exerts its functions and how organisms discriminate cytosine hydroxymethylation from methylation...........more PDF
 Nanoscale arrangement of proteins by Single-Molecule Cut & Paste
Nanoscale arrangement of proteins by Single-Molecule Cut & Paste
Strackharn M., Pippig D.A., Meyer P., Stahl S.W., Gaub H.E.
JACS, 134(37), 15193-6 (2012)
Protein-based nanostructures are key to the organization of life and it is their precise arrangement, which determines their specific functions. A single-molecule approach for the directed assembly of protein arrangements allows for a controlled composition of systems based on protein components. Applying antibodies and .......more PDF

Single-Molecule Adhesion of a Stimuli-Responsive Oligo(ethylene glycol)
Copolymer to Gold
Michael A. Nash and Hermann E. Gaub
ACS Nano, 6 (12), pp 10735–10742 (2012)
Adhesion of environmentally responsive polymers to biocompatible surfaces is an important issue that has been explored in several nanobiotechnology.......more PDF
 DNA–Protein Binding Force Chip
DNA–Protein Binding Force Chip
Philip M. D. Severin and Hermann E. Gaub
Nano Micro Small 8, No. 21, 3269–3273 (2012)
Force measurements provide new fundamental and complementary information on biomolecular interactions, particularly in the high and low affi nity regimes, which may hardly be obtained otherwise..............more PDF
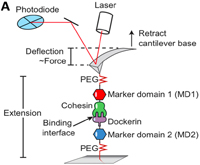
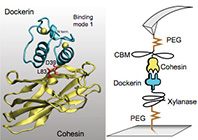 Single-molecule dissection of the high-affinity cohesin–dockerin complex
Single-molecule dissection of the high-affinity cohesin–dockerin complex
Stefan W. Stahl, Michael A. Nash, Daniel B. Fried, Michal Slutzki, Yoav Barak, Edward A. Bayer and Hermann E. Gaub
PNAS 109 , No. 50, 20431–20436 (2012)
Cellulose-degrading enzyme systems are of significant interest from both a scientific and technological perspective due to the diversity of cellulase families, their unique.....more PDF
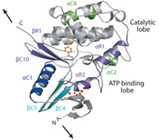 Single-Molecule Mechanoenzymatics
Single-Molecule Mechanoenzymatics
Elias M. Puchner and Hermann E. Gaub
Annual Review Biophysics 41, 497-518 (2012)
The ability of cellular signaling networks to sense, process, and respond to internal and external stimuli relies on their specific detection and trans- duction based on molecular recognition. The molecular mechanisms by which force ............. more PDF
Functional Assembly of Aptamer Binding Sites by Single-MoleculeCut-and-Paste
Mathias Strackharn, Stefan W. Stahl, Elias M. Puchner and Hermann E. Gaub
Nano Lett. 12, 2425-2428 (2012)
Bottom up assembly of functional molecular ensembles with novel properties emerging from composition and arrangement of ......more PDF
 Peptide–Antibody Complex as Handle for Single-Molecule Cut & Paste
Peptide–Antibody Complex as Handle for Single-Molecule Cut & Paste
Mathias Strackharn, Stefan W. Stahl, Philip M. D. Severin, Thomas Nicolaus and Hermann E. Gaub
ChemPhysChem, 13, Issue 4, 914–917 (2012)
Feynman is frequently quoted for having foreseen that individ- ual atoms may be arranged one-by-one to form functional as- semblies. The seminal work by Don Eigler and colleagues convincingly proved the validity of these concepts...........more PDF
Severin PMD, Ho D, Gaub HE (2011).
A high throughput molecular force assay for protein-DNA interactions.
Lab on a Chip, 11 (5):856-862. PDF
Dufrene YF, Evans E, Engel A, Helenius J, Gaub HE, Muller DJ (2011).
Five challenges to bringing single-molecule force spectroscopy into living cells.
Nature Methods, 8 (2):123-127. PDF
Erdmann M, David R, Fornof AR, Gaub HE (2011).
Electric Glue: Electrically Controlled Polymer-Surface Adhesion.
Nano Letters, 11 (5):1993-1996. PDF
Stahl SW, Puchner EM, Alexandrovich A, Gautel M, Gaub HE (2011).
A conditional gating mechanism assures the integrity of the molecular force-sensor titin kinase.
Biophys J., 101 (8):1978-86." PDF
Te Riet J, Katan AJ, Rankl C, Stahl SW, van Buul AM, Phang IY, Gomez-Casado A, Schön P, Gerritsen JW, Cambi A, Rowan AE, Vancso GJ, Jonkheijm P, Huskens J, Oosterkamp TH, Gaub H, Hinterdorfer P, Figdor CG, Speller S. (2011).
Interlaboratory round robin on cantilever calibration for AFM force spectroscopy.
Ultramicroscopy, 111(12):1659-1669. PDF
Pippig DA, Heucke SF, Klamecka K, Kufer SK, Severin PM, Stahl SW, Strackharn M, Gaub HE (2011)
Single molecule cut and paste for protein based functional assembly.
European Biophysics Journal with Biophysics Letters, 40:p. 221-221
Severin Philip M. D., Zou Xueqing, Gaub Hermann E., et al. (2011).
Cytosine methylation alters DNA mechanical properties.
Nucleic Acids Research, 39 (20):8740-8751 PDF
Puchner EM, Gaub HE (2010).
Exploring the Conformation-Regulated Function of Titin Kinase by Mechanical Pump and Probe Experiments with Single Molecules.
Angewandte Chemie-International Edition, 49 (6):1147-1150. PDF
Erdmann M, David R, Fornof A, Gaub HE (2010).
Electrically controlled DNA adhesion.
Nature Nanotechnology, 5 (2):154-159.
Cordes T, Strackharn M, Stahl SW, Summerer W, Steinhauer C, Forthmann C, Puchner EM, Vogelsang J, Gaub HE, Tinnefeld P (2010).
Resolving Single-Molecule Assembled Patterns with Superresolution Blink-Microscopy.
Nano Letters 10 (2):645-651.
Erdmann M, David R, Fornof AR, Gaub HE (2010).
Electrically induced bonding of DNA to gold.
Nature Chemistry, 2 (9):745-749.
Puchner EM, Gaub HE, Editor(s) Graslund A, Rigler R, Widengren J, (2010).
Mechanoenzymatics and Nanoassembly of Single Molecules.
Single Molecule Spectroscopy in Chemistry, Physics and Biology p:289-303.
Vogelsang J, Cordes T, Forthmann C, Steinhauer C, Tinnefeld P (2010).
Intrinsically Resolution Enhancing Probes for Confocal Microscopy.
Nano Letters, 10(2):672-679.
Cordes T, Vogelsang J, Anaya M, Spagnuolo C, Gietl A, Summerer W, Herrmann A, Mullen K, Tinnefeld P (2010).
Single-Molecule Redox Blinking of Perylene Diimide Derivatives in Water.
Journal of the American Chemical Society, 132(7):2404-2409.
Spalthoff C, Egelhaaf M, Tinnefeld P, Kurtz R (2010).
Localized direction selective responses in the dendrites of visual interneurons of the fly.
BMC Biology, 8 Art No. 36.
Kasper R, Harke B, Forthmann C, Tinnefeld P, Hell SW, Sauer M (2010).
Single-Molecule STED Microscopy with Photostable Organic Fluorophores.
Small, 6(13):1379-1384.
Vogelsang J, Steinhauer C, Forthmann C, Stein IH, Person-Skegro B, Cordes T, Tinnefeld P (2010).
Make them Blink: Probes for Super-Resolution Microscopy.
Chemphyschem, 11(12):2475-2490.
Jungmann R, Steinhauer C, Scheible M, Kuzyk A, Tinnefeld P, Simmel FC (2010).
Single-Molecule Kinetics and Super-Resolution Microscopy by Fluorescence Imaging of Transient Binding on DNA Origami
Nano Letters, 10(11):4756-4761.
Kufer SK, Strackharn M, Stahl SW, Gumpp H, Puchner EM, Gaub HE (2009).
Optically monitoring the mechanical assembly of single molecules.
Nature Nanotechnology 4 (1):45-49.
Ho D, Falter K, Severin P, Gaub HE (2009).
DNA as a Force Sensor in an Aptamer-Based Biochip for Adenosine.
Analytical Chemistry 81 (8):3159-3164.
Ho D, Dose C, Albrecht CH, Severin P, Falter K, Dervan PB, Gaub HE (2009).
Quantitative Detection of Small Molecule/DNA Complexes Employing a Force-Based and Label-Free DNA-Microarray.
Biophysical Journal 96 (11):4661-4671.
Gumpp H, Stahl SW, Strackharn M, Puchner EM, Gaub HE (2009).
Ultrastable combined atomic force and total internal fluorescence microscope.
Review of Scientific Instruments 80 (6): Art No. 063704.
Stahl SW, Puchner EM, Gaub HE (2009).
Photothermal cantilever actuation for fast single-molecule force spectroscopy.
Review of Scientific Instruments, 80(7):Art No. 073702.
Gumpp H, Puchner EM, Zimmermann JL, Gerland U, Gaub HE, Blank K (2009).
Triggering Enzymatic Activity with Force.
Nano Letters 9 (9):3290-3295.
Gaub BM, Kaul C, Zimmermann JL, Carell T, Gaub HE (2009).
Switching the mechanics of dsDNA by Cu salicylic aldehyde complexation.
Nanotechnology, 20 (43): Art No. 434002.
Gumpp H, Stahl SW, Strackharn M, Puchner EM, Gaub HE (2009).
Ultrastable combined atomic force and total internal fluorescence microscope
(vol 80, 063704, 2009).Review of Scientific Instruments, 80 (10): Art No. 109901.
Puchner EM, Gaub HE (2009).
Force and function: probing proteins with AFM-based force spectroscopy.
Current Opinion in Structural Biology, 19 (5):605-614.
Ho D, Zimmermann JL, Dehmelt FA, Steinbach U, Erdmann M, Severin P, Falter K, Gaub HE (2009).
Force-Driven Separation of Short Double-Stranded DNA.
Biophysical Journal 97 (12):3158-3167.
Vogelsang J, Cordes T, Tinnefeld P (2009).
Single-molecule photophysics of oxazines on DNA and its application in a FRET switch.
Photochemical& Photobiological Sciences, 8(4):486-496.
Vogelsang J, Cordes T, Tinnefeld P (2009).
On the Mechanism of Trolox as Antiblinking and Antibleaching Reagent.
Journal of the American Chemical Society, 131(14):5018.
Vogelsang J, Cordes T, Forthmann C, Steinhauer C, Tinnefeld P (2009).
Controlling the fluorescence of ordinary oxazine dyes for single-molecule switching and superresolution microscopy.
Proceedings of the National Academy of Sciences of the United States of America, 106(20):8107-8112.
Person B, Stein IH, Steinhauer C, Vogelsang J, Tinnefeld P (2009).
Correlated Movement and Bending of Nucleic Acid Structures Visualized by Multicolor Single-Molecule Spectroscop.
Chemphyschem, 10(9-10):1455-1460.
Steinhauer C, Jungmann R, Sobey TL, Simmel FC, Tinnefeld P (2009).
DNA Origami as a Nanoscopic Ruler for Super-Resolution Microscopy.
Angewandte Chemie-International Edition, 48(47):8870-8873.
Kufer SK, Puchner EM, Gumpp H, Liedl T, Gaub HE (2008).
Single-molecule cut-and-paste surface assembly.
Science, 319 (5863):594-596
Helenius J, Heisenberg CP, Gaub HE, Muller DJ (2008).
Single-cell force spectroscopy.
Journal of Cell Science, 121 (11):1785-1791
Albrecht CH, Neuert G, Lugmaier RA, Gaub HE (2008).
Molecular force balance measurements reveal that double-stranded DNA unbinds under force in rate-dependent pathways.
Biophysical Journal, 94 (12):4766-4774
Sonnenberg L, Billon L, Gaub HE (2008).
Competitive adhesion reduces the effective bridging length of polymers.
Macromolecules, 41 (10):3688-3691
Engel A, Gaub HE (2008).
Structure and mechanics of membrane proteins.
Annual Review of Biochemistry, 77127-148
Puchner EM, Franzen G, Gautel M, Gaub HE (2008).
Comparing proteins by their unfolding pattern.
Biophysical Journal,95 (1):426-434
Morfill J, Neumann J, Blank K, Steinbach U, Puchner EM, Gottschalk KE, Gaub HE (2008).
Force-based analysis of multidimensional energy landscapes: Application of dynamic force spectroscopy and steered molecular dynamics simulations to an antibody fragment-peptide complex.
Journal of Molecular Biology, 381 (5):1253-1266
Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brandmeier B, Grater F, Grubmuller H, Gaub HE, Gautel M (2008).
Mechanoenzymatics of titin kinase.
Proceedings of the National Academy of Sciences of the united States of America, 105 (36):13385-13390
David R, Ho D, Gaub HE (2008).
Molecular force balance for the investigation of receptor-ligand-interactions on living cells.
Journal pf Peptide Science, 14 (8):134-134
Puchner EM, Kufer SK, Strackharn M, Stahl SW, Gaub HE (2008).
Nanoparticle Self-Assembly on a DNA-Scaffold Written by Single-Molecule Cut-and-Paste.
Nano Letters, 8 (11):3692-3695
Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brandmeier B, Grater F, Grubmuller H, Gaub HE, Gautel M (2008).
Mechanoenzymatics of titin kinase
(vol 105, pg 13385, 2008).Proceedings of the National Academy of Sciences of the United States of America, 105 (52):21045-21045
Vogelsang J, Kasper R, Steinhauer C, Person B, Heilemann M, Sauer M, Tinnefeld P (2008).
A reducing and oxidizing system minimizes photobleaching and blinking of fluorescent dyes.
Angewandte Chemie-International Edition, 47(29):5465-5469
Heilemann M, van de Linde S, Schuttpelz M, Kasper R, Seefeldt B, Mukherjee A, Tinnefeld P, Sauer M (2008).
Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes.
Angewandte Chemie-International Edition, 47 (33):6172-6176
Huang S, Xiao Q, He ZK, Liu Y, Tinnefeld P, Su XR, Peng XN (2008).
A high sensitive and specific QDs FRET bioprobe for MNase.
Chemical Communications, (45):5990-5992
Seefeldt B, Kasper R, Seidel T, Tinnefeld P, Dietz KJ, Heilemann M, Sauer M (2008).
Fluorescent proteins for single-molecule fluorescence applications.
Journal of Biophotonics, 1(1):74-82
Steinhauer C, Forthmann C, Vogelsang J, Tinnefeld P (2008).
Superresolution Microscopy on the Basis of Engineered Dark States.
Journal of the American Chemical Society, 130(50):16840
Schmitz J, Gottschalk KE (2008).
Mechanical regulation of cell adhesion.
Soft Matter, 4(7):1373-1387
Schmitz J, Benoit M, Gottschalk KE (2008).
The viscoelasticity of membrane tethers and its importance for cell adhesion.
Biophysical Journal, 95(3):1448-1459
Kuhner F, Lugmaier RA, Mihatsch S, Gaub HE (2007).
Print your atomic force microscope.
Review of Scientific Instruments, 78(7): Art N° 075105
Kuhner F, Morfill J, Neher RA, Blank K, Gaub HE (2007).
Force-induced DNA slippage.
Biophysical Journal, 92(7):2491-2497
Morfill J, Kuhner F, Blank K, Lugmaier RA, Sedlmair J, Gaub HE (2007).
B-S transition in short oligonucleotides.
Biophysical Journal, 93(7):2400-2409
Neuert G, Albrecht CH, Gaub HE (2007).
Predicting the rupture probabilities of molecular bonds in series.
Biophysical Journal, 93 (4):1215-1223
Schafer LV, Muller EM, Gaub HE, Grubmuller H (2007).
Elastic properties of photoswitchable azobenzene polymers from molecular dynamics simulations.
Angewandte Chemie-International Edition, 46 (13):2232-2237
Sonnenberg L, Parvole J, Kuhner F, Billon L, Gaub HE (2007).
Choose sides: Differential polymer adhesion.
Langmuir, 23 (12):6660-6666
Morfill J, Blank K, Zahnd C, Luginbuhl B, Kuhner F, Gottschalk KE, Pluckthun A, Gaub HE (2007).
Affinity-matured recombinant antibody fragments analyzed by single-molecule force Spectroscopy.
Biophysical Journal, 933583-3590
Dose C, Ho D, Gaub HE, Dervan PB, Albrecht CH (2007).
Recognition of "mirror-image" DNA by small molecules.
Angewandte Chemie-International Edition, 468384-8387
Cui SX, Yu J, Kuhner F, Schulten K, Gaub HE (2007).
Double-stranded DNA dissociates into single strands when dragged into a poor solvent.
Journal of the American Chemical Society, 12914710-14716
Sonnenberg L, Luo YF, Schlaad H, Seitz M, Colfen H, Gaub HE (2007).
Quantitative single molecule measurements on the interaction forces of poly(L-glutamic acid) with calcite crystals.
Journal of the American Chemical Society,129 (49):15364-15371
Vogelsang J, Doose S, Sauer M, Tinnefeld P (2007).
Single-molecule fluorescence resonance energy transfer in nanopipets: Improving distance resolution and concentration range.
Analytical Chemistry 797367-7375
Albrecht, Clausen-Schaumnn H, Gaub HE (2006).
Differential analysis of biomolecular forces.
Journal of Physics-Condensed Matter, 18(18):S581–S599.
Blank K, Morfill J, Gumpp H, Gaub HE (2006).
Functional expression of Candida antarctica lipase B in Eschericha coli.
J of Biotechnology, 125(4):474-483.
Blank K, Morfill J, Gaub HE (2006).
Site-specific immobilization of genetically engineered variants of Candida antartica lipase B.
Chembiochem, 7(9):1349–1351.
Cui SX, Albrecht C, Kuhner F, Gaub HE (2006).
Weakly bound water molecules shorten single-stranded DNA.
J of the American Chemical Society, 128(20):6636-6639.
Friedsam C, Gaub HE, Netz RR (2006).
Probing surfaces with single-polymer atomic force microscope ex periments.
BIOINTERPHASES, 1(1):MR1-MR21.
Kessler M, Gaub HE(2006).
Unfolding barriers in bacteriorhodopsin probed from the cytoplasmic and the extracellular side by AFM.
Structure, 14(3):521-527.
Kessler M, Gottschalk KE, Janovjak H, Muller DJ, Gaub HE (2006).
Bacteriorhodopsin folds into the membrane against an external force.
J of Molecular Biology, 357(2):644–654.
Kuhner F, Erdmann M, Sonnenberg L, Serr A, Morfill J, Gaub HE (2006).
Friction of single polymers at surfaces.
Langmuir, 22(26):11180–11186.
Kuhner F, Gaub HE (2006).
Modelling cantilever-based force spectroscopy with polymers.
Polymer, 47(7):2555–2563.
Kuhner F, Erdmann M, Gaub HE (2006).
Scaling exponent and Kuhn length of pinned polymers by single molecule force spectroscopy.
Physical Review Letters, 97 Art No. 218301.
Neuert G, Albrecht C, Pamir E, Gaub HE (2006).
Dynamic force spectroscopy of the digoxigenin-antibody complex.
Febs Letters, 580(2):505–509.
Neuert G, Hugel T, Netz RR, Gaub HE (2006).
Elasticity of poly(azobenzene-peptides).
Macromolecules, 39(2):789-797. PDF
Sonnenberg L, Parvole J, Borisov O, Billon L, Gaub HE, Seitz M (2006).
AFM-based single molecule force spectroscopy of end-grafted poly(acrylic acid) monolayers.
Macromolecules, 39(1):281-288.
Friedsam C, Gaub H, and Netz R (2005).
Adsorption energies of single charged polymers.
Europhysics Letters, 72(5):844–850.
Füzesi M, Gottschalk KE, Lindzen M, Shainskaya A, Küster B, Garty H, and Karlish SJ (2005).
Covalent cross-links between the gamma subunit (FXYD2) and alpha and beta subunits of Na,K-ATPase: modeling the alpha-gamma interaction.
J Biol Chem, 280(18):18291–18301.
Gottschalk KE (2005).
A coiled-coil structure of the alphaIIbbeta3 integrin transmembrane and cytoplasmic domains in its resting state.
Structure, 13(5):703–712.
Heilemann M, Margeat E, Kasper R, Sauer M, and Tinnefeld P (2005).
Carbocyanine dyes as efficient reversible single-molecule optical switch.
J Am Chem Soc, 127(11):3801-3806.
Heinlein T, Biebricher A, Schluter P, Roth CM, Herten DP, Wolfrum J, Heilemann M, Muller C, Tinnefeld P, and Sauer M (2005).
High-resolution colocalization of single molecules within the resolution gap of far-field microscopy.
ChemPhysChem, 6(5):949-955.
Hugel T, Rief M, Seitz M, Gaub H, and Netz R (2005).
Highly stretched single polymers: Atomic-force-microscope experiments versus ab-initio theory.
Physical Review Letters, 94(4):048301.
Kirchner C, Liedl T, Kudera S, Pellegrino T, Javier A, Gaub H, Stolzle S, Fertig N, and Parak W (2005).
Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles.
Nano Letters, 5(2):331–338.
Kufer S, Dietz H, Albrecht C, Blank K, Kardinal A, Rief M, and Gaub H (2005).
Covalent immobilization of recombinant fusion proteins with hAGT for single molecule force spectroscopy.
European Biophysics Journal With Biophysics Letters, 35(1):72–78.
Lugmaier R, Hugel T, Benoit M, and Gaub H (2005).
Phase contrast and DIC illumination for AFM hybrids.
Ultramicroscopy, 104(3-4):255–260.
Neuert G, Kufer S, Benoit M, and Gaub H (2005).
Modular multichannel surface plasmon spectrometer.
Review Of Scientific Instruments, 76(5):054303.
Strugatsky D, Gottschalk KE, Goldshleger R, and Karlish SJ (2005).
D443 of the N domain of Na+,K+-ATPase interacts with the ATP-Mg2+ complex, possibly via a second Mg2+ ion.
Biochemistry, 44(49):15961–15969.
Tinnefeld P, and Sauer M (2005).
Branching out of single-molecule fluorescence spectroscopy: challenges for chemistry and influence on biology.
Angew Chem Int Ed, 44(18):2642-2671.
Tinnefeld P, Heilemann M, and Sauer M (2005).
Design of molecular photonic wires based on multistep electronic excitation transfer.
ChemPhysChem, 6(2):217-222.
Bell TD, Habuchi S, Masuo S, Österling I, Müllen K, Tinnefeld P, Sauer M, van der Auweraer M, Hofkens J, and De Schryver F (2004).
Single photon emission from a dendrimer containing eight perylene diimide chromophores.
Aust J Chem, 57(12):1169-1173.
Biebricher A, Paul A, Tinnefeld P, Gölzhäuser A, and Sauer M (2004).
Controlled three-dimensional immobilization of biomolecules on chemically patterned surfaces.
J Biotechnol, 112(1-2):97-107.
Blank K, Lankenau A, Mai T, Schiffmann S, Gilbert I, Hirler S, Albrecht C, Benoit M, Gaub H, and Clausen-Schaumann H (2004).
Double-chip protein arrays: force-based multiplex sandwich immunoassays with increased specificity.
Analytical And Bioanalytical Chemistry, 379(7-8):974–981.
Friedsam C, Becares A, Jonas U, Seitz M, and Gaub H (2004).
Adsorption of polyacrylic acid on self-assembled monolayers investigated by single-molecule force spectroscopy.
New Journal Of Physics, 6:9.
Friedsam C, Seitz M, and Gaub H (2004).
Investigation of polyelectrolyte desorption by single molecule force spectroscopy.
Journal Of Physics-Condensed Matter, 16(26):S2369–S2382.
Gilbert L, Schiffmann S, Rubenwolf S, Jensen K, Mail T, Albrecht C, Lankenau A, Beste G, Blank K, Gaub H, and Clausen-Schaumann H (2004).
Double chip protein arrays using recombinant single-chain Fv antibody fragments.
Proteomics, 4(5):1417–1420.
Gottschalk KE (2004).
Structure prediction of small transmembrane helix bundles.
J Mol Graph Model, 23(1):99–110.
Gottschalk KE, and Kessler H (2004).
A computational model of transmembrane integrin clustering.
Structure, 12(6):1109–1116.
Gottschalk KE, and Kessler H (2004).
Evidence for hetero-association of transmembrane helices of integrins.
FEBS Lett, 557(1-3):253–258.
Gottschalk KE, Neuvirth H, and Schreiber G (2004).
A novel method for scoring of docked protein complexes using predicted protein-protein binding sites.
Protein Eng Des Sel, 17(2):183–189.
Gottschalk KE, Soskine M, Schuldiner S, and Kessler H (2004).
A structural model of EmrE, a multi-drug transporter from Escherichia coli.
Biophys J, 86(6):3335–3348.
Heilemann M, Tinnefeld P, Sanchez Mosteiro G, Garcia Parajo M, Van Hulst N, and Sauer M (2004).
Multistep energy transfer in single molecular photonic wires.
J Am Chem Soc, 126(21):6514-6515.
Herten DP, Biebricher A, Heilemann M, Heinlein T, Müller C, Schlüter P, Tinnefeld P, Weston K, Sauer M, and Wolfrum J (2004).
Optical single molecule techniques for analytical and biological applications.
Rec Res Devel Phys Chem, 7:345-368 (invited article).
Kuhner F, Costa L, Bisch P, Thalhammer S, Heckl W, and Gaub H (2004).
LexA-DNA bond strength by single molecule force spectroscopy.
Biophysical Journal, 87(4):2683–2690.
Marinelli L, Gottschalk K, Meyer A, Novellino E, and Kessler H (2004).
Human integrin alphavbeta5: homology modeling and ligand binding.
J Med Chem, 47(17):4166–4177.
Stein B, George M, Gaub H, and Parak W (2004).
Extracellular measurements of averaged ionic currents with the light-addressable potentiometric sensor (LAPS).
Sensors And Actuators B-Chemical, 98(2-3):299–304.
Tinnefeld P, Buschmann V, Weston K, Biebricher A, Herten DP, Piestert O, Heinlein T, Heilemann M, and Sauer M (2004).
How single-molecule photophysical studies complement ensemble methods for a better understanding of chromophores and interchromophore interactions.
Rec Res Devel Phys Chem, 7:95-125 (invited article).
Tinnefeld P, Hofkens J, Herten DP, Masuo S, Vosch T, Cotlet M, Habuchi S, Mullen K, De Schryver F, and Sauer M (2004).
Higher-excited-state photophysical pathways in multichromophoric systems revealed by single-molecule fluorescence spectroscopy.
ChemPhysChem, 5(11):1786-1790.
Vardy E, Arkin I, Gottschalk K, Kaback HR, and Schuldiner S (2004).
Structural conservation in the major facilitator superfamily as revealed by comparative modeling.
Protein Sci, 13(7):1832–1840.
Albrecht C, Blank K, Lalic-Multhaler M, Hirler S, Mai T, Gilbert I, Schiffmann S, Bayer T, Clausen-Schaumann H, and Gaub H (2003).
DNA: A programmable force sensor.
Science, 301(5631):367–370.
Blank K, Mai T, Gilbert I, Schiffmann S, Rankl J, Zivin R, Tackney C, Nicolaus T, Spinnler K, Oesterhelt F, Benoit M, Clausen-Schaumann H, and Gaub H (2003).
A force-based protein biochip.
Proceedings Of The National Academy Of Sciences Of The United States Of America, 100(20):11356–11360.
Friedsam C, Wehle A, Kuhner F, and Gaub H (2003).
Dynamic single-molecule force spectroscopy: bond rupture analysis with variable spacer length.
Journal Of Physics-Condensed Matter, 15(18):S1709–S1723.
Hofkens J, Cotlet M, Vosch T, Tinnefeld P, Weston K, Ego C, Grimsdale A, Mullen K, Beljonne D, Bredas JL, Jordens S, Schweitzer G, Sauer M, and De Schryver F (2003).
Revealing competitive Forster-type resonance energy-transfer pathways in single bichromophoric molecules.
Proc Natl Acad Sci USA, 100(23):13146-13151.
Holland N, Hugel T, Neuert G, Cattani-Scholz A, Renner C, Oesterhelt D, Moroder L, Seitz M, and Gaub H (2003).
Single molecule force spectroscopy of azobenzene polymers: Switching elasticity of single photochromic macromolecules.
Macromolecules, 36(6):2015–2023.
Krautbauer R, Rief M, and Gaub H (2003).
Unzipping DNA oligomers.
Nano Letters, 3(4):493–496.
Kudera M, Eschbaumer C, Gaub H, and Schubert U (2003).
Analysis of metallo-supramolecular systems using single-molecule force spectroscopy.
Advanced Functional Materials, 13(8):615–620.
Marinelli L, Lavecchia A, Gottschalk K, Novellino E, and Kessler H (2003).
Docking studies on alphavbeta3 integrin ligands: pharmacophore refinement and implications for drug design.
J Med Chem, 46(21):4393–4404.
Seitz M, Friedsam C, Jostl W, Hugel T, and Gaub H (2003).
Probing solid surfaces with single polymers.
Chemphyschem, 4(9):986–990.
Stein B, George M, Gaub H, Behrends J, and Parak W (2003).
Spatially resolved monitoring of cellular metabolic activity with a semiconductor-based biosensor.
Biosensors & Bioelectronics, 18(1):31–41.
Strugatsky D, Gottschalk KE, Goldshleger R, Bibi E, and Karlish SJ (2003).
Expression of Na+,K+-ATPase in Pichia pastoris: analysis of wild type and D369N mutant proteins by Fe2+-catalyzed oxidative cleavage and molecular modeling.
J Biol Chem, 278(46):46064–46073.
Tinnefeld P, Buschmann V, Weston K, and Sauer M (2003).
Direct Observation of collective blinking and energy transfer in a bichromophoric system.
J Phys Chem A, 107(3):323-327.
Vosch T, Cotlet M, Hofkens J, van der Biest K, Lor M, Weston K, Tinnefeld P, Sauer M, Latterini L, Müllen K, and De Schryver F (2003).
Probing Förster Type Energy Pathways in a first generation rigid dendrimer bearing two perylene imide chromophores.
J Phys Chem A, 107(36):6920-6931.
Benoit M, and Gaub H (2002).
Measuring cell adhesion forces with the atomic force microscope at the molecular level.
Cells Tissues Organs, 172(3):174–189.
Gottschalk K, and Kessler H (2002).
The structures of integrins and integrin-ligand complexes: implications for drug design and signal transduction.
Angew Chem Int Ed Engl, 41(20):3767–3774.
Gottschalk K, Adams P, Brunger A, and Kessler H (2002).
Transmembrane signal transduction of the alpha(IIb)beta(3) integrin.
Protein Sci, 11(7):1800–1812.
Gottschalk K, Günther R, and Kessler H (2002).
A three-state mechanism of integrin activation and signal transduction for integrin alpha(v)beta(3).
Chembiochem, 3(5):470–473.
Heilemann M, Herten D, Heintzmann R, Cremer C, Muller C, Tinnefeld P, Weston K, Wolfrum J, and Sauer M (2002).
High-resolution colocalization of single dye molecules by fluorescence lifetime imaging microscopy.
Anal Chem, 74(14):3511-3517.
Hugel T, Holland N, Cattani A, Moroder L, Seitz M, and Gaub H (2002).
Single-molecule optomechanical cycle.
Science, 296(5570):1103–1106.
Kirchner C, George M, Stein B, Parak W, Gaub H, and Seitz M (2002).
Corrosion protection and long-term chemical functionalization of gallium arsenide in an aqueous environment.
Advanced Functional Materials, 12(4):266–276.
Krautbauer R, Fischerlander S, Allen S, and Gaub H (2002).
Mechanical fingerprints of DNA drug complexes.
Single Molecules, 3(2-3):97–103.
Krautbauer R, Pope L, Schrader T, Allen S, and Gaub H (2002).
Discriminating small molecule DNA binding modes by single molecule force spectroscopy.
Febs Letters, 510(3):154–158.
Krautbauer R, Schrader T, Pope L, Allen S, and Gaub H (2002).
Discriminating small molecule DNA binding modes by single molecule force spectroscopy.
Biophysical Journal, 82(1):141A–142A.
Tinnefeld P, Weston K, Vosch T, Cotlet M, Weil T, Hofkens J, Mullen K, De Schryver F, and Sauer M (2002).
Antibunching in the emission of a single tetrachromophoric dendritic system.
J Am Chem Soc, 124(48):14310-14311.
Urry D, Hugel T, Seitz M, Gaub H, Sheiba L, Dea J, Xu J, and Parker T (2002).
Elastin: a representative ideal protein elastomer.
Philosophical Transactions Of The Royal Society Of London Series B-Biological Sciences, 357(1418):169–184.
Weston K, Dyck M, Tinnefeld P, Muller C, Herten D, and Sauer M (2002).
Measuring the number of independent emitters in single-molecule fluorescence images and trajectories using coincident photons.
Anal Chem, 74(20):5342-5349.
Gaub H (2001).
Unfolding bacteriorhodopsin and unbinding CSA by AFM-related techniques.
Abstracts Of Papers Of The American Chemical Society, 221:U324–U324.
Krautbauer R, Allen S, Tessmer I, Skinner G, Pope L, Schrader T, Molloy J, Tendler SJ, and Gaub H (2001).
Single molecule force spectroscopy investigations of DNA-drug interactions.
Abstracts Of Papers Of The American Chemical Society, 221:U345–U345.
Parak W, George M, Kudera R, Gaub H, and Behrends J (2001).
Effects of semiconductor substrate and glia-free culture on the development of voltage-dependent currents in rat striatal neurones.
European Biophysics Journal With Biophysics Letters, 29(8):607–620.
Tinnefeld P, Herten DP, and Sauer M (2001).
Photophysical dynamics of single molecules studied by spectrally-resolved fluorescence lifetime-imaging microscopy (FLIM).
J Phys Chem A, 105(34):7989-8003.
Tinnefeld P, Müller C, and Sauer M (2001).
Time-varying photon probability distribution of individual molecules at room temperature.
Chem Phys Lett, 345(3-4):252-258.
Benoit M, Gabriel D, Gerisch G, and Gaub H (2000).
Discrete interactions in cell adhesion measured by single-molecule force spectroscopy.
Nature Cell Biology, 2(6):313–317.
Bohm S, Parak W, George M, Gaub H, and Lorke A (2000).
Characterization of the field-effect addressable potentiometric sensor (FAPS).
Sensors And Actuators B-Chemical, 68(1-3):266–273.
Clausen-Schaumann H, Rief M, Tolksdorf C, and Gaub H (2000).
Mechanical stability of single DNA molecules.
Biophysical Journal, 78(4):1997–2007.
Clausen-Schaumann H, Seitz M, Krautbauer R, and Gaub H (2000).
Force spectroscopy with single bio-molecules.
Current Opinion In Chemical Biology, 4(5):524–530.
Dettmann W, Grandbois M, Andre S, Benoit M, Wehle A, Kaltner H, Gabius H, and Gaub H (2000).
Differences in zero-force and force-driven kinetics of ligand dissociation from beta-galactoside-specific proteins (plant and animal lectins, immunoglobulin G) monitored by plasmon resonance and dynamic single molecule force microscopy.
Archives Of Biochemistry And Biophysics, 383(2):157–170.
Gaub H (2000).
Single molecule force spectroscopy with AFM-related techniques (Langmuir lecture).
Abstracts Of Papers Of The American Chemical Society, 220:U260–U260.
George M, Parak W, and Gaub H (2000).
Highly integrated surface potential sensors.
Sensors And Actuators B-Chemical, 69(3):266–275.
George M, Parak W, Gerhardt I, Moritz W, Kaesen F, Geiger H, Eisele I, and Gaub H (2000).
Investigation of the spatial resolution of the light-addressable potentiometric sensor.
Sensors And Actuators A-Physical, 86(3):187–196.
Grandbois M, Dettmann W, Benoit M, and Gaub H (2000).
Affinity imaging of red blood cells using an atomic force microscope.
Journal Of Histochemistry & Cytochemistry, 48(5):719–724.
Herten DP, Tinnefeld P, and Sauer M (2000).
Identification of single fluorescently labelled mononucleotide molecules in solution by spectrally resolved time-correlated single-photon counting.
Appl Phys B, 71:765-771.
Irngartinger H, Fettel PW, Escher T, Tinnefeld P, Nord S, and Sauer M (2000).
Substituent effects on redox properties and photoinduced electron transfer in isoxazolo-fullerenes.
Eur J Org Chem, 3:455-465.
Krautbauer R, Clausen-Schaumann H, and Gaub H (2000).
Cisplatin changes the mechanics of single DNA molecules.
Angewandte Chemie-International Edition, 39(21):3912–+.
Oesterhelt F, Oesterhelt D, Pfeiffer M, Engel A, Gaub H, and Muller D (2000).
Unfolding pathways of individual bacteriorhodopsins.
Science, 288(5463):143–146.
Parak W, Dannohl S, George M, Schuler M, Schaumburger J, Gaub H, Muller O, and Aicher W (2000).
Metabolic activation stimulates acid production in synovial fibroblasts.
Journal Of Rheumatology, 27(10):2312–2322.
Parak W, George M, Domke J, Radmacher M, Behrends J, Denyer M, and Gaub H (2000).
Can the light-addressable potentiometric sensor (LAPS) detect extracellular potentials of cardiac myocytes?
Ieee Transactions On Biomedical Engineering, 47(8):1106–1113.
Rief M, Gautel M, and Gaub H (2000).
Unfolding forces of titin and fibronectin domains directly measured by AFM.
Elastic Filaments Of The Cell, 481:129–141.
Schmitt L, Ludwig M, Gaub H, and Tampe R (2000).
A metal-chelating microscopy tip as a new toolbox for single-molecule experiments by atomic force microscopy.
Biophysical Journal, 78(6):3275–3285.
Tinnefeld P, Buschmann V, Herten DP, Han KT, and Sauer M (2000).
Confocal fluorescence lifetime imaging microscopy (FLIM) at the single molecule level.
Single Mol, 1(3):215-223.
Zhang W, Xu Q, Zou S, Li H, Xu W, Zhang X, Shao Z, Kudera M, and Gaub H (2000).
Single-molecule force spectroscopy on Bombyx mori silk fibroin by atomic force microscopy.
Langmuir, 16(9):4305–4308.

