Contact
und Center for NanoScience
Ludwig-Maximilians-Universität
am Max-Planck-Institut für Biochemie
Am Klopferspitz 18
82152 Martinsried
Room:
m 332
Phone:
+49 (0) 89/ 2180-3133
Email:
martin.benoit@physik.uni-muenchen.de
Office hours:
on arrangement
Responsibilities
advanced practical courses & assistant researcher
topics:
cell mechanics
membrane forces
cell adhesion forces
inter- and intra-molecular forces
methods:
force measurements (AFM, MT)
planar patch-clamp
Further Information
Publications:
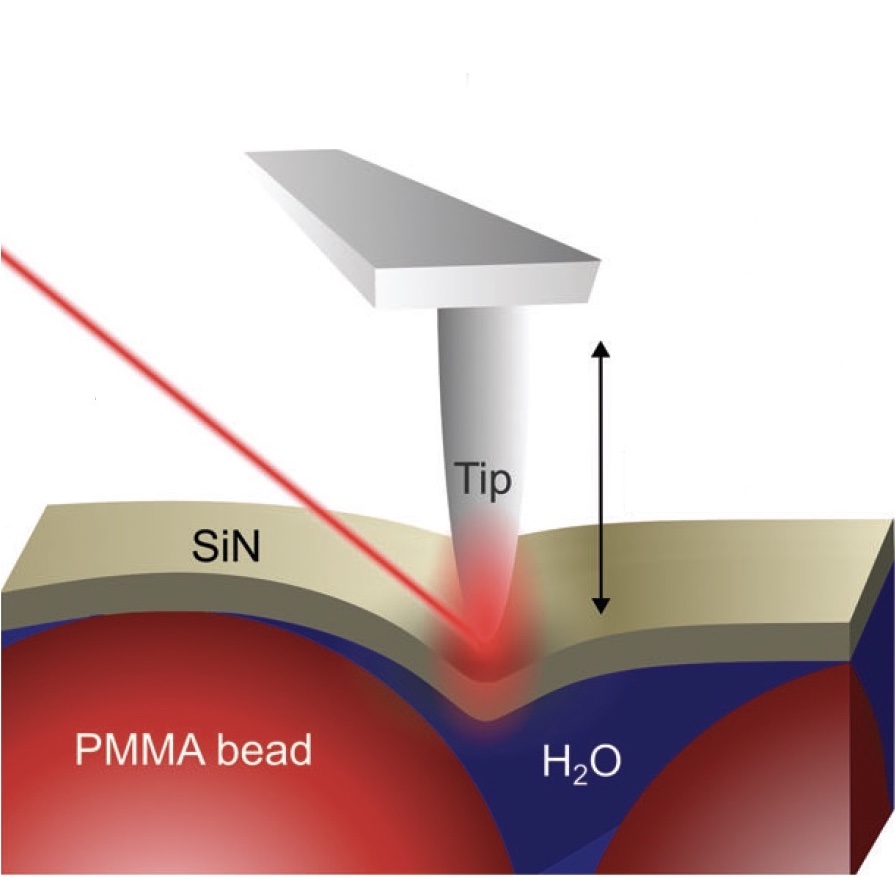 Nanoscale Mechanical Manipulation of Ultrathin SiN Membranes Enabling Infrared Near-Field Microscopy of Liquid-Immersed samples
Nanoscale Mechanical Manipulation of Ultrathin SiN Membranes Enabling Infrared Near-Field Microscopy of Liquid-Immersed samples
Enrico Baù, Thorsten Gölz, Martin Benoit, Andreas Tittl and Fritz Keilmann
Small, November 2024, doi: 10.1002/smll.202402568
Scattering scanning near-field optical microscopy (s-SNOM) is a powerful technique for mid-infrared spectroscopy at nanometer length scales. By investigating objects in aqueous environments through ultrathin membranes, s-SNOM has recently been extended towards label-free nanoscopy of the dynamics of living cells and nanoparticles, assessing both the optical and the mechanical interactions between the tip, the membrane and the liquid suspension underneath... more PDF
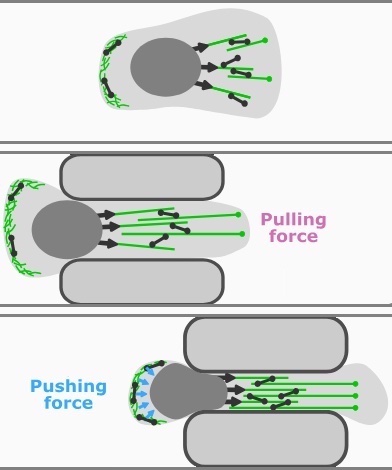 Nuclear deformation and dynamics of migrating cells in 3D confinement reveal adaptation of pulling and pushing forces
Nuclear deformation and dynamics of migrating cells in 3D confinement reveal adaptation of pulling and pushing forces
Stefan Stöberl, Johannes Flommersfeld, Maximilian M. Kreft, Martin Benoit, Chase P. Broedersz and Joachim O. Rädler
Science Advances, August 2024, doi: 10.1182/bloodadvances.2022006978
Eukaryotic cells show an astounding ability to remodel their shape and cytoskeleton and to migrate through pores and constrictions smaller than their nuclear diameter. However, the relation of nuclear deformation and migration dynamics in confinement remains unclear. Here, we study the mechanics and dynamics of mesenchymal cancer cell nuclei transitioning through three-dimensional compliant hydrogel channels. We find a biphasic dependence of migration speed and transition frequency on channel width... more PDF
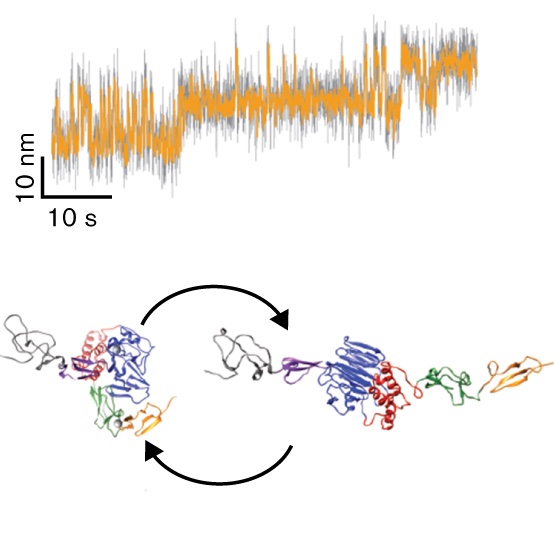 A conformational transition of the D'D3 domain primes von Willebrand factor for multimerization
A conformational transition of the D'D3 domain primes von Willebrand factor for multimerization
Sophia Gruber, Achim Löf, Adina Hausch, Fabian Kutzki, Res Jöhr, Tobias Obser, Gesa König, Reinhard Schneppenheim, Camilo Aponte-Santamaría, Frauke Gräter, Maria A. Brehm, Martin Benoit and Jan Lipfert
Blood Advances, September 2022, doi: 10.1182/bloodadvances.2022006978
Von Willebrand factor (VWF) is a multimeric plasma glycoprotein that is critically involved in hemostasis. Biosynthesis of long VWF concatemers in the endoplasmic reticulum and the trans-Golgi is still not fully understood. We use the single-molecule force spectroscopy technique magnetic tweezers to analyze a previously hypothesized conformational change in the D'D3 domain crucial for VWF multimerization. We find that... more PDF
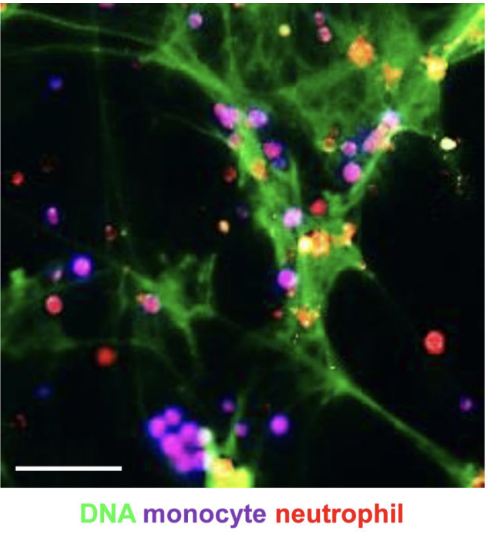 Endotoxinemia Accelerates Atherosclerosis Through Electrostatic Charge-
Endotoxinemia Accelerates Atherosclerosis Through Electrostatic Charge-
Mediated Monocyte Adhesion.
Ariane Schumski, A. Ortega-Gómez, K. Wichapong, C. Winter, P. Lemnitzer, J. R Viola, M. Pinilla-Vera, E. Folco, V. Solis-Mezarino, M. Völker-Albert, S. L Maas, C. Pan, L. Perez Olivares, J. Winter, T. Hackeng, M. C I Karlsson, T. Zeller, A. Imhof, R. M Baron, G. A F Nicolaes, P. Libby, L. Maegdefessel, F. Kamp, M. Benoit, Y. Döring and O. Soehnlein
Circulation. Januar 2021, doi:10.1161/CIRCULATIONAHA.120.046677
Acute infection is a well-established risk factor of cardiovascular inflammation increasing the risk for a cardiovascular complication within the first weeks after infection. However, the nature of the processes underlying such aggravation remains unclear. Lipopolysaccharide derived from Gram-negative bacteria is a potent activator of circulating immune cells including neutrophils, which foster inflammation through discharge of neutrophil extracellular traps (NETs)... more PDF
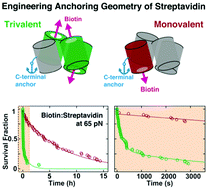
Designed anchoring geometries determine lifetimes of biotin–streptavidin bonds under constant load and enable ultra-stable coupling
Sophia Gruber, Achim Löf, Steffen M. Sedlak, M. Benoit, Hermann E. Gaub and Jan Lipfert
Nanoscale, October 2020, DOI: 10.1039/d0nr03665j
The small molecule biotin and the homotetrameric protein streptavidin (SA) form a stable and robust complex that plays a pivotal role in many biotechnological and medical applications. In particular, the SA–biotin linkage is frequently used in single-molecule force spectroscopy (SMFS) experiments. Recent data suggest that SA–biotin bonds show strong directional dependence and a broad range of multi-exponential lifetimes under load. Here, we investigate engineered SA variants... more PDF
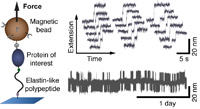 Multiplexed protein force spectroscopy reveals equilibrium protein folding dynamics and the low-force response of von Willebrand factor
Multiplexed protein force spectroscopy reveals equilibrium protein folding dynamics and the low-force response of von Willebrand factor
Achim Löf, Philipp U. Walker, Steffen M. Sedlak, Sophia Gruber, Tobias Obser, Maria A. Brehm, M. Benoit, Jan Lipfert
PNAS, August 2019, doi.org/10.1073/pnas.1901794116
Single-molecule force spectroscopy has provided unprecedented insights into protein folding, force regulation, and function. So far, the field has relied primarily on atomic force microscope and optical tweezers assays that, while powerful, are limited in force resolution, throughput, and require feedback for constant force measurements. Here, we present a modular approach based on magnetic tweezers (MT) for highly multiplexed protein force spectroscopy. Our approach uses elastin-like polypeptide linkers for the specific attachment of proteins, requiring only short peptide tags on the protein of interest. The assay extends protein force spectroscopy into the low force (<1 pN) regime and enables parallel and ultra-stable measurements at constant forces... more PDF
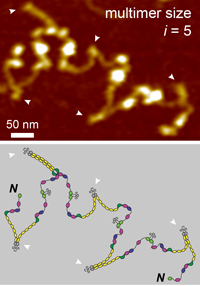 Advancing multimer analysis of von Willebrand factor by single-molecule AFM imaging
Advancing multimer analysis of von Willebrand factor by single-molecule AFM imaging
Achim Löf, Gesa König, Sonja Schneppenheim, Reinhard Schneppenheim, M. Benoit, Ulrich Budde, Jochen P. Müller, and Maria A. Brehm
PLoS ONE 2019, DOI http://dx.plos.org/10.1371/journal.pone.0210963
The formation of hemostatic plugs at sites of vascular injury crucially involves the multimeric glycoprotein von Willebrand factor (VWF). VWF multimers are linear chains of N-terminally linked dimers. The latter are formed from monomers via formation of the C-terminal disulfide bonds Cys2771-Cys2773’, Cys2773-Cys2771’, and Cys2811-Cys2811’. Mutations in VWF that impair multimerization can lead to subtype 2A of the bleeding disorder von Willebrand Disease (VWD). Commonly, the multimer size distribution of VWF is assessed by electrophoretic multimer analysis. Here, we present atomic force microscopy (AFM) imaging as a method to determine the size distribution of VWF variants by direct visualization at the single-molecule level. We first validated our approach by investigating recombinant wildtype VWF and a previously studied mutant (p.Cys1099Tyr) that impairs N-terminal multimerization... more PDF
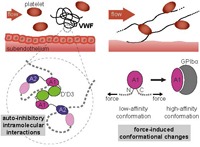 A Biophysical View on von Willebrand Factor Activation
A Biophysical View on von Willebrand Factor Activation
Achim Löf, Jochen P. Müller, and Maria A. Brehm,
Journal of Cellular Physiology, March 2017, doi: 10.1002/jcp.25887,
The process of hemostatic plug formation at sites of vascular injury crucially relies on the large multimeric plasma glycoprotein von Willebrand factor (VWF) and its ability to recruit platelets to the damaged vessel wall via interaction of its A1 domain with platelet GPIbα. Under normal blood flow conditions, VWF multimers exhibit a very low binding affinity for platelets. Only when subjected to increased hydrodynamic forces, which primarily occur in connection with vascular injury, VWF can efficiently bind to platelets. This force-regulation of VWF’s hemostatic activity is not only highly intriguing from a biophysical perspective, but also of eminent physiological importance... more PDF
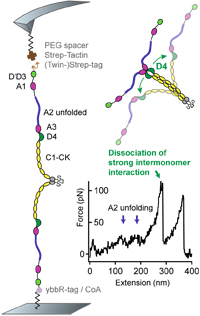 Biophysical approaches promote advances in the understanding of von Willebrand factor processing and function
Biophysical approaches promote advances in the understanding of von Willebrand factor processing and function
Achim Löf, Jochen P. Müller, M. Benoit, Maria A. Brehm
Advances in Biological Regulation 2017, 63:81-91
doi: 10.1016/j.jbior.2016.09.010.
The large multimeric plasma glycoprotein von Willebrand factor (VWF) is essential for primary hemostasis by recruiting platelets to sites of vascular injury. VWF multimers respond to elevated hydrodynamic forces by elongation, thereby increasing their adhesiveness to platelets. Thus, the activation of VWF is force-induced, as is its inactivation. Due to these attributes, VWF is a highly interesting system from a biophysical point of view, and is well suited for investigation using biophysical approaches. Here, we give an overview on recent studies that predominantly employed biophysical methods to gain novel insights into multiple aspects of VWF: Electron microscopy was used to shed light on the domain structure of VWF and the mechanism of VWF secretion... more PDF
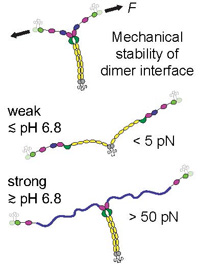 pH-Dependent Interactions in Dimers Govern the Mechanics and Structure of von Willebrand Factor
pH-Dependent Interactions in Dimers Govern the Mechanics and Structure of von Willebrand Factor
Jochen P. Müller, Achim Löf, Salomé Mielke, Tobias Obser, Linda K. Bruetzel, Willem Vanderlinden, Jan Lipfert, Reinhard Schneppenheim, and M. Benoit,
Biophysical Journal, July 2016 111,2,p312–322, doi:10.1016/j.bpj.2016.06.022
Von Willebrand factor (VWF) is a multimeric plasma glycoprotein that is activated for hemostasis by increased hydrodynamic forces at sites of vascular injury. Here, we present data from atomic force microscopy-based single-molecule force measurements, atomic force microscopy imaging, and small-angle x-ray scattering to show that the structure and mechanics of VWF are governed by multiple pH-dependent interactions with opposite trends within dimeric subunits. In particular, the recently discovered strong intermonomer interaction, which induces a firmly closed conformation of dimers and crucially involves the D4 domain... more PDF
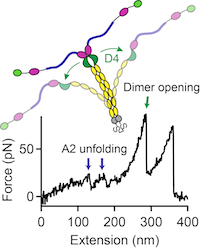
Force sensing by the vascular protein von Willebrand factor is tuned by a strong intermonomer interaction
Jochen P. Müller, Salomé Mielke, Achim Löf, Tobias Obser, Christof Beer, Linda K. Bruetzel, Diana A. Pippig, Willem Vanderlinden, Jan Lipfert, Reinhard Schneppenheim, M. Benoit
PNAS, January 2016 doi: 10.1073/pnas.1516214113
The large plasma glycoprotein von Willebrand factor (VWF) senses hydrodynamic forces in the bloodstream and responds to elevated forces with abrupt elongation, thereby increasing its adhesiveness to platelets and collagen. Remarkably, forces on VWF are elevated at sites of vascular injury, where VWF’s hemostatic potential is important to mediate platelet aggregation and to recruit platelets to the subendothelial layer... more PDF
 Decoding Cytoskeleton-Anchored and Non-Anchored Receptors from Single-Cell Adhesion Force Data
Decoding Cytoskeleton-Anchored and Non-Anchored Receptors from Single-Cell Adhesion Force Data
Ediz Sariisik, Cvetan Popov, Jochen P. Müller, Denitsa Docheva, Hauke Clausen-Schaumann and M. Benoit
Biophys J. 2015 Oct 6;109(7):1330-3. doi: 10.1016/j.bpj.2015.07.048
Complementary to parameters established for cell-adhesion force curve analysis, we evaluated the slope before a force step together with the distance from the surface at which the step occurs and visualized the result in a two-dimensional density plot. This new tool allows detachment steps of long membrane tethers to be distinguished from shorter jumplike force steps.... more PDF
 A fast recoiling silk-like elastomer facilitates nanosecond nematocyst discharge.
A fast recoiling silk-like elastomer facilitates nanosecond nematocyst discharge.
Beckmann A, Xiao S, Müller JP, Mercadante D, Nüchter T, Kröger N, Langhojer F, Petrich W, Holstein T, Benoit M, Gräter F, Özbek S.
BMC Biol. 2015 Jan 16;13:3. doi: 10.1186/s12915-014-0113-1
The discharge of the Cnidarian stinging organelle, the nematocyst, is one of the fastest processes in biology and involves volume changes of the highly pressurised (150 bar) capsule of up to 50%. Hitherto, the molecular basis for the unusual biomechanical properties of nematocysts has been elusive, as their structure was mainly defined as a stress-resistant collagenous matrix. Here, we characterise Cnidoin, a novel elastic protein... more PDF
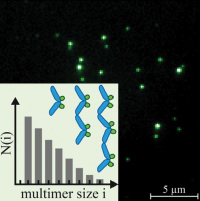 Exponential size distribution of von Willebrand factor.
Exponential size distribution of von Willebrand factor.
Lippok S, Obser T, Müller JP, Stierle VK, Benoit M, Budde U, Schneppenheim R, Rädler JO.
Biophys J. 2013 Sep 3;105(5):1208-16. doi: 10.1016/j.bpj.2013.07.037.
Von Willebrand Factor (VWF) is a multimeric protein crucial for hemostasis. Under shear flow, it acts as a mechanosensor responding with a size-dependent globule-stretch transition to increasing shear rates. Here, we quantify for the first time, to our knowledge, the size distribution of recombinant VWF and VWF-eGFP using a multilateral approach that involves quantitative gel analysis, fluorescence correlation spectroscopy, and total internal reflection fluorescence microscopy... more PDF
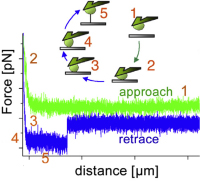 A practical guide to quantify cell adhesion using single-cell force spectroscopy.
A practical guide to quantify cell adhesion using single-cell force spectroscopy.
Friedrichs J, Legate KR, Schubert R, Bharadwaj M, Werner C, Müller DJ, Benoit M.
Methods. 2013 Apr 1;60(2):169-78. doi: 10.1016/j.ymeth.2013.01.006
Quantitative analysis of cellular interactions with the extracellular environment is necessary to gain an understanding of how cells regulate adhesion in the development and maintenance of multicellular organisms, and how changes in cell adhesion contribute to diseases. We provide a practical guide to quantify the adhesive strength of living animal cells to various substrates using atomic force microscopy (AFM)-based single-cell force spectroscopy (SCFS)... more PDF
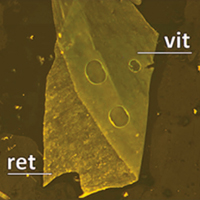 Vital dyes increase the rigidity of the internal limiting membrane
Vital dyes increase the rigidity of the internal limiting membrane
Haritoglou C, Mauell S, Benoit M, Schumann RG, Henrich PB, Wolf A and Kampik A
Eye advance online publication, 16 August 2013; doi:10.1038/eye.2013.178
To assess the stiffness of the natural human internal limiting membrane (ILM) and evaluate potential changes of the mechanical properties following staining with brilliant blue (BB) and indocyanine green (ICG).
Methods Unstained ILM specimens were obtained during ophthalmic surgical procedures. After removal, the specimens were dissected into five parts. .... more PDF
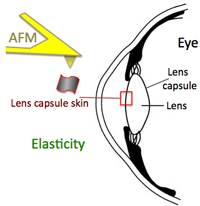 Increase in lens capsule stiffness caused by vital dyes
Increase in lens capsule stiffness caused by vital dyes
Christos Haritoglou, Stephan Mauell, Ricarda G. Schumann, Paul B. Henrich, Armin Wolf, Marcus Kernt and M. Benoit
J Cataract Refract Surg 2013; 39:1749–1752 Q 2013 ASCRS and ESCRS, doi:10.1016/j.jcrs.2013.02.057
Cataract surgery was among the first ophthalmic surgical procedures in which dyes were introduced to assist the surgeon in more challenging cases, such as eyes with mature cataract. In mature white cataracts, a controlled capsulorhexis of the anterior capsule is often difficult to perform due to absence of the red fundus reflex. .... more PDF
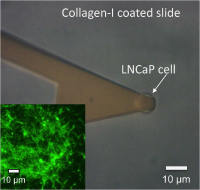 Probing the interaction forces of prostate cancer cells with collagen I and bone marrow derived stem cells on the single cell level.
Probing the interaction forces of prostate cancer cells with collagen I and bone marrow derived stem cells on the single cell level.
Sarıısık E, Docheva D, Padula D, Popov C, Opfer J, Schieker M, Clausen-Schaumann H, Benoit M.
Published: March 5, 2013 DOI: 10.1371/journal.pone.0057706
Adhesion of metastasizing prostate carcinoma cells was quantified for two carcinoma model cell lines LNCaP (lymph node-specific) and PC3 (bone marrow-specific). By time-lapse microscopy and force spectroscopy we found PC3 cells to preferentially adhere to bone marrow-derived mesenchymal stem cells (SCP1 cell line)... more PDF
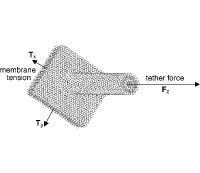 Measuring cell adhesion forces: theory and principles.
Measuring cell adhesion forces: theory and principles.
M. Benoit and Christine Selhuber-Unkel
Methods Mol Biol;736:355-77. PubMed PMID: 21660737.
Cell adhesion is an essential prerequisite for survival, communication, and navigation of cells in organisms. It is maintained by the organized binding of molecules from the cell membrane to the extracellular space. This chapter focuses on direct measurements of cellular binding strength at the level of single adhesion molecules... more PDF
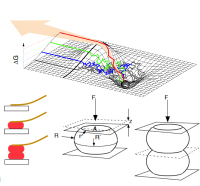 Force Spectroscopy on Cells.
Force Spectroscopy on Cells.
M. Benoit, 2011
Chapter 9, in: handbook of nanophysics 7; 9:1-29; ISBN 978-1-4200-7546-5
... Physicists say: “a cell…” while biologists say, “we took an endothelial cell from
the upper third of the dorsal endometrium in the early S2-phase of a 12 days old male…” For a cell-adhesion measurement with molecular resolution, the biophysicist has a dilemma. The information from the individual adhesion molecule is embedded in the concert of all the participating molecules of the cell including
the membrane and the cytoskeleton... PDF
2009 Cuerrier CM, Benoit M, Guillemette G, Gobeil F Jr, Grandbois M.
Real-time monitoring of angiotensin II-induced contractile response and cytoskeleton remodeling in individual cells by atomic force microscopy.
Pflugers Arch;457(6):1361-72.
PubMed PMID: 18953565. PDF
2008 Schmitz J, Benoit M and Gottschalk KE,
The viscoelasticity of membrane tethers and its importance for cell adhesion.
Biophys J, 95(3):1448–1459
PubMed PMID: 18456832 PDF
2008 Pamir E, George M, Fertig N, Benoit M.
Planar patch-clamp force microscopy on living cells.
Ultramicroscopy;108(6):552-7.
PubMed PMID: 17933465. PDF
2007 Lugmaier RA, Schedin S, Kühner F, Benoit M.
Dynamic restacking of Escherichia coli P-pili.
Eur Biophys J;37(2):111-20.
PubMed PMID: 17554533. PDF
2006 Muñoz Javier A, Kreft O, Piera Alberola A, Kirchner C, Zebli B, Susha AS, Horn E, Kempter S, Skirtach AG, Rogach AL, Rädler J, Sukhorukov GB, Benoit M, Parak WJ.
Combined atomic force microscopy and optical microscopy measurements as a method to investigate particle uptake by cells.
Small;2(3):394-400.
PubMed PMID: 17193058. PDF
2006 Nüchter T, Benoit M, Engel U, Ozbek S, Holstein TW
Nanosecond-scale kinetics of nematocyst discharge.
Curr Biol;16(9):R316-8
PMID:16682335 PDF
2005 Lugmaier R, Hugel T, Benoit M and Gaub HE,
Phase contrast and DIC illumination for AFM hybrids.
Ultramicroscopy, 104(3-4):255–260
PubMed PMID: 15961230. PDF
2005 Alon R, Feigelson SW, Manevich E, Rose DM, Schmitz J, Overby DR, Winter E, Grabovsky V, Shinder V, Matthews BD, Sokolovsky-Eisenberg M, Ingber DE, Benoit M, Ginsberg MH.J
Alpha4beta1-dependent adhesion strengthening under mechanical strain is regulated by paxillin association with the alpha4-cytoplasmic domain.
Cell Biol;171(6):1073-84.
PubMed PMID: 16365170. PDF
2005 Neuert G, Kufer S, Benoit M and Gaub HE,
Modular multichannel surface plasmon spectrometer.
Review Of Scientific Instruments, 76(5):054303. PDF
2004 Blank K, Lankenau A, Mai T, Schiffmann S, Gilbert I, Hirler S, Albrecht C, Benoit M, Gaub HE and Clausen-Schaumann H,
Double-chip protein arrays: force-based multiplex sandwich immunoassays with increased specificity.
Analytical And Bioanalytical Chemistry, 379(7-8):974–981.
PubMed PMID: 15103448. PDF
2003 Blank K, Mai T, Gilbert I, Schiffmann S, Rankl J, Zivin R, Tackney C, Nicolaus T, Spinnler K, Oesterhelt F, Benoit M, Clausen-Schaumann H and Gaub HE,
A force-based protein biochip.
Proceedings Of The National Academy Of Sciences Of The United States Of America, 100(20):11,356–11360
PubMed PMID: 12975526; PDF
2002 Benoit M.
Cell adhesion measured by force spectroscopy on living cells.
Methods Cell Biol;68:91-114.
PubMed PMID: 12053742. PDF
2002 Benoit M and Gaub HE
Measuring cell adhesion forces with the atomic force microscope at the molecular level.
Cells Tissues Organs, 172(3):174–189
PubMed PMID: 12476047. PDF
2000 Benoit M, Gabriel D, Gerisch G and Gaub HE,
Discrete interactions in cell adhesion measured by single-molecule force spectroscopy.
Nature Cell Biology, 2(6):313–317
PubMed PMID: 10854320. PDF
2000 Dettmann W, Grandbois M, Andre S, Benoit M, Wehle A, Kaltner H, Gabius H and Gaub HE,
Differences in zero-force and force-driven kinetics of ligand dissociation from beta-galactoside-specific proteins (plant and animal lectins, immunoglobulin G) monitored by plasmon resonance and dynamic single molecule force microscopy.
Archives Of Biochemistry And Biophysics, 383(2):157–170
PubMed PMID: 11185549. PDF
2000 Grandbois M, Dettmann W, Benoit M and Gaub HE,
Affinity imaging of red blood cells using an atomic force microscope.
Journal Of Histochemistry & Cytochemistry, 48(5):719–724
PubMed PMID: 10769056. PDF
1998 Thie M, Rospel R, Dettmann W, Benoit M, Ludwig M, Gaub HE and Denker H,
Interactions between trophoblast and uterine epithelium: monitoring of adhesive forces.
Human Reproduction, 13(11):3211–3219
PubMed PMID: 9853883. PDF
1997 Benoit M, Holstein T and Gaub HE,
Lateral forces in AFM imaging and immobilization of cells and organelles.
European Biophysics Journal With Biophysics Letters, 26(4):283–290.
PubMed PMID: 17838043. PDF
1994 Holstein TW, Benoit M, Herder vG, Wanner G, David CN, Gaub HE
Fibrous Mini-Collagens in Hydra Nematocysts.
Science, 265(5170):402-4
PMID:17838043 PDF


